Concordance of DMET plus genotyping results with those of orthogonal genotyping methods
- PMID: 22871999
- PMCID: PMC3516299
- DOI: 10.1038/clpt.2012.95
Concordance of DMET plus genotyping results with those of orthogonal genotyping methods
Abstract
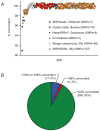
There are several hurdles to the clinical implementation of pharmacogenetics. One approach is to employ pre-prescription genotyping, involving interrogation of multiple pharmacogenetic variants using a high-throughput platform. We compared the performance of the Drug Metabolizing Enzymes and Transporters (DMET) Plus array (1,931 variants in 225 genes) with that of orthogonal genotyping methods in 220 pediatric patients. A total of 1,692 variants had call rates >98% and were in Hardy-Weinberg equilibrium. Of these, 259 were genotyped by at least one independent method, and a total of 19,942 single-nucleotide polymorphism (SNP)-patient sample pairs were evaluated. The concordance rate was 99.9%, with only 28 genotype discordances observed. For the genes deemed most likely to be clinically relevant (TPMT, CYP2D6, CYP2C19, CYP2C9, VKORC1, DPYD, UGT1A1, and SLCO1B1), a total of 3,799 SNP-patient sample pairs were evaluable and had a concordance rate of 99.96%. We conclude that the DMET Plus array performs well with primary patient samples, with the results in good concordance with those of several lower-throughput genotyping methods.
Conflict of interest statement
M.V.R and W.E.E: receive a portion of the income St. Jude receives from licensing patent rights related to TPMT and GGH polymorphisms.
Figures

Comment in
-
DMET™ Plus array delivers results in good concordance with those of several lower-throughput genotyping methods in patient samples.Pharmacogenomics. 2013 Feb;14(3):238-9. Pharmacogenomics. 2013. PMID: 23527392 No abstract available.
References
-
- Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–49. - PubMed
-
- Kalow W, Tang BK, Endrenyi L. Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283–9. - PubMed
-
- Dervieux T, Meshkin B, Neri B. Pharmacogenetic testing: proofs of principle and pharmacoeconomic implications. Mutat Res. 2005;573:180–94. - PubMed
-
- Sakurai A, Tamura A, Onishi Y, Ishikawa T. Genetic polymorphisms of ATP-binding cassette transporters ABCB1 and ABCG2: therapeutic implications. Expert Opin Pharmacother. 2005;6:2455–73. - PubMed
-
- Deeken J. The Affymetrix DMET platform and pharmacogenetics in drug development. Curr Opin Mol Ther. 2009;11:260–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

