A novel 3D mouse embryo atlas based on micro-CT
- PMID: 22872090
- PMCID: PMC6514304
- DOI: 10.1242/dev.082016
A novel 3D mouse embryo atlas based on micro-CT
Abstract
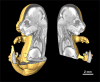
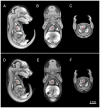
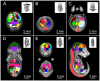
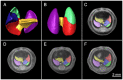
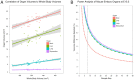
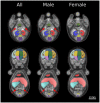
The goal of the International Mouse Phenotyping Consortium (IMPC) is to phenotype targeted knockout mouse strains throughout the whole mouse genome (23,000 genes) by 2021. A significant percentage of the generated mice will be embryonic lethal; therefore, phenotyping methods tuned to the mouse embryo are needed. Methods that are robust, quantitative, automated and high-throughput are attractive owing to the numbers of mice involved. Three-dimensional (3D) imaging is a useful method for characterizing morphological phenotypes. However, tools to automatically quantify morphological information of mouse embryos from 3D imaging have not been fully developed. We present a representative mouse embryo average 3D atlas comprising micro-CT images of 35 individual C57BL/6J mouse embryos at 15.5 days post-coitum. The 35 micro-CT images were registered into a consensus average image with our automated image registration software and 48 anatomical structures were segmented manually. We report the mean and variation in volumes for each of the 48 segmented structures. Mouse organ volumes vary by 2.6-4.2% on a linear scale when normalized to whole body volume. A power analysis of the volume data reports that a 9-14% volume difference can be detected between two classes of mice with sample sizes of eight. This resource will be crucial in establishing baseline anatomical phenotypic measurements for the assessment of mutant mouse phenotypes, as any future mutant embryo image can be registered to the atlas and subsequent organ volumes calculated automatically.
Figures
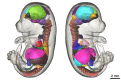


References
-
- Bock N. A., Konyer N. B., Henkelman R. M. (2003). Multiple-mouse MRI. Magn. Reson. Med. 49, 158–167. - PubMed
-
- Cleary J. O., Modat M., Norris F. C., Price A. N., Jayakody S. A., Martinez-Barbera J. P., Greene N. D. E., Hawkes D. J., Ordidge R. J., Scambler P. J., et al. (2011). Magnetic resonance virtual histology for embryos: 3D atlases for automated high-throughput phenotyping. Neuroimage 54, 769–778. - PubMed
-
- Collins D. L., Neelin P., Peters T. M., Evans A. C. (1994). Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 18, 192–205. - PubMed
-
- Copp A. J. (1995). Death before birth: clues from gene knockouts and mutations. Trends Genet. 11, 87–93. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

