D-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems
- PMID: 22872108
- PMCID: PMC3555687
- DOI: 10.1007/s00726-012-1364-1
D-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems
Abstract
D-Aspartate (D-Asp) is an endogenous amino acid in the central nervous and reproductive systems of vertebrates and invertebrates. High concentrations of D-Asp are found in distinct anatomical locations, suggesting that it has specific physiological roles in animals. Many of the characteristics of D-Asp have been documented, including its tissue and cellular distribution, formation and degradation, as well as the responses elicited by D-Asp application. D-Asp performs important roles related to nervous system development and hormone regulation; in addition, it appears to act as a cell-to-cell signaling molecule. Recent studies have shown that D-Asp fulfills many, if not all, of the definitions of a classical neurotransmitter-that the molecule's biosynthesis, degradation, uptake, and release take place within the presynaptic neuron, and that it triggers a response in the postsynaptic neuron after its release. Accumulating evidence suggests that these criteria are met by a heterogeneous distribution of enzymes for D-Asp's biosynthesis and degradation, an appropriate uptake mechanism, localization within synaptic vesicles, and a postsynaptic response via an ionotropic receptor. Although D-Asp receptors remain to be characterized, the postsynaptic response of D-Asp has been studied and several L-glutamate receptors are known to respond to D-Asp. In this review, we discuss the current status of research on D-Asp in neuronal and neuroendocrine systems, and highlight results that support D-Asp's role as a signaling molecule.
Figures

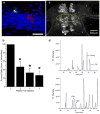
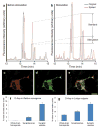
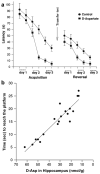
References
-
- Abe K, Takahashi S, Muroki Y, Kera Y, Yamada RH. Cloning and expression of the pyridoxal 5′-phosphate-dependent aspartate racemase gene from the bivalve mollusk Scapharca broughtonii and characterization of the recombinant enzyme. J Biochem. 2006;139(2):235–244. doi: 10.1093/jb/mvj028. - DOI - PubMed
-
- Adachi M, Koyama H, Long ZQ, Sekine M, Furuchi T, Imai K, Nimura N, Shimamoto K, Nakajima T, Homma H. L-glutamate in the extracellular space regulates endogenous D-aspartate homeostasis in rat pheochromocytoma MPT1 cells. Arch Biochem Biophys. 2004;424(1):89–96. doi: 10.1016/j.abb.2004.01.016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

