Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance
- PMID: 22872213
- PMCID: PMC5233443
- DOI: 10.1007/s00125-012-2665-3
Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance
Abstract
Aims/hypothesis: Inflammation in obesity increases the levels of the suppressor of cytokine signalling-3 (SOCS3) protein in adipose tissue, but the physiological importance of this protein in regulating whole-body insulin sensitivity in obesity is not known.
Methods: We generated Socs3 floxed (wild-type, WT) and Socs3 aP2 (also known as Fabp4)-Cre null (Socs3 AKO) mice. Mice were maintained on either a regular chow or a high-fat diet (HFD) for 16 weeks during which time body mass, adiposity, glucose homeostasis and insulin sensitivity were assessed.
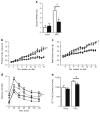
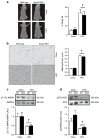
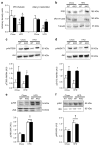
Results: The HFD increased SOCS3 levels in adipose tissue of WT but not Socs3 AKO mice. WT and Socs3 AKO mice had similar body mass and adiposity, assessed using computed tomography (CT) imaging, irrespective of diet or sex. On a control chow diet there were no differences in insulin sensitivity or glucose tolerance. When fed a HFD, female but not male Socs3 AKO mice had improved glucose tolerance as well as lower fasting glucose and insulin levels compared with WT littermates. Hyperinsulinaemic-euglycaemic clamps and positron emission tomography (PET) imaging demonstrated that improved insulin sensitivity was due to elevated adipose tissue glucose uptake. Increased insulin-stimulated glucose uptake in adipose tissue was associated with enhanced levels and activating phosphorylation of insulin receptor substrate-1 (IRS1).
Conclusions/interpretation: These data demonstrate that inhibiting SOCS3 production in adipose tissue of female mice is effective for improving whole-body insulin sensitivity in obesity.
Conflict of interest statement
Duality of interest The authors declare there is no duality of interest associated with this manuscript.
Figures
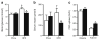
References
-
- Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279:821–824. - PubMed
-
- Mori H, Hanada R, Hanada T, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. - PubMed
-
- Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. - PubMed
-
- Steinberg GR, Smith AC, Wormald S, Malenfant P, Collier C, Dyck DJ. Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E57–E63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

