Alterations in lipid signaling underlie lipodystrophy secondary to AGPAT2 mutations
- PMID: 22872237
- PMCID: PMC3478532
- DOI: 10.2337/db12-0004
Alterations in lipid signaling underlie lipodystrophy secondary to AGPAT2 mutations
Erratum in
- Diabetes. 2013 Mar;62(3):998. Elliot, Brandon [corrected to Elliott, Brandon G]
Abstract
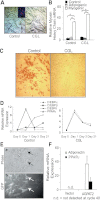
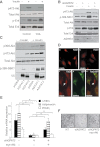
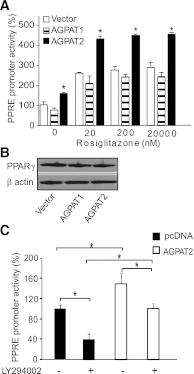
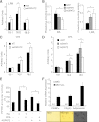
Congenital generalized lipodystrophy (CGL), secondary to AGPAT2 mutation is characterized by the absence of adipocytes and development of severe insulin resistance. In the current study, we investigated the adipogenic defect associated with AGPAT2 mutations. Adipogenesis was studied in muscle-derived multipotent cells (MDMCs) isolated from vastus lateralis biopsies obtained from controls and subjects harboring AGPAT2 mutations and in 3T3-L1 preadipocytes after knockdown or overexpression of AGPAT2. We demonstrate an adipogenic defect using MDMCs from control and CGL human subjects with mutated AGPAT2. This defect was rescued in CGL MDMCs with a retrovirus expressing AGPAT2. Both CGL-derived MDMCs and 3T3-L1 cells with knockdown of AGPAT2 demonstrated an increase in cell death after induction of adipogenesis. Lack of AGPAT2 activity reduces Akt activation, and overexpression of constitutively active Akt can partially restore lipogenesis. AGPAT2 modulated the levels of phosphatidic acid, lysophosphatidic acid, phosphatidylinositol species, as well as the peroxisome proliferator-activated receptor γ (PPARγ) inhibitor cyclic phosphatidic acid. The PPARγ agonist pioglitazone partially rescued the adipogenic defect in CGL cells. We conclude that AGPAT2 regulates adipogenesis through the modulation of the lipome, altering normal activation of phosphatidylinositol 3-kinase (PI3K)/Akt and PPARγ pathways in the early stages of adipogenesis.
Figures



References
-
- Huang-Doran I, Sleigh A, Rochford JJ, O’Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol 2010;207:245–255 - PubMed
-
- Agarwal AK, Arioglu E, De Almeida S, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 2002;31:21–23 - PubMed
-
- Miranda DM, Wajchenberg BL, Calsolari MR, et al. Novel mutations of the BSCL2 and AGPAT2 genes in 10 families with Berardinelli-Seip congenital generalized lipodystrophy syndrome. Clin Endocrinol (Oxf) 2009;71:512–517 - PubMed
-
- Leung DW. The structure and functions of human lysophosphatidic acid acyltransferases. Front Biosci 2001;6:D944–D953 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

