Spermatogenesis
- PMID: 22872478
- PMCID: PMC9217098
- DOI: 10.1007/978-1-4614-4015-4_7
Spermatogenesis
Abstract
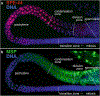
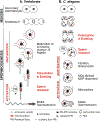
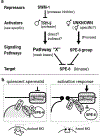
During spermatogenesis, pluripotent germ cells differentiate to become efficient delivery vehicles to the oocyte of paternal DNA. Though male and female germ cells both undergo meiosis to produce haploid complements of DNA, at the same time they also each undergo distinct differentiation processes that result in either sperm or oocytes. This review will discuss our current understanding of mechanisms of sperm formation and differentiation in Caenorhabditis elegans gained from studies that employ a combination of molecular, transcriptomic, and cell biological approaches. Many of these processes also occur during spermatogenesis in other organisms but with differences in timing, molecular machinery, and morphology. In C. elegans, sperm differentiation is implemented by varied modes of gene regulation, including the genomic organization of genes important for sperm formation, the generation of sperm-specific small RNAs, and the interplay of specific transcriptional activators. As sperm formation progresses, chromatin is -systematically remodeled to allow first for the implementation of differentiation programs, then for sperm-specific DNA packaging required for transit of paternal genetic and epigenetic information. Sperm also exhibit distinctive features of -meiotic progression, including the formation of a unique karyosome state and the centrosomal-based segregation of chromosomes during symmetric meiotic -divisions. Sperm-specific organelles are also assembled and remodeled as cells complete -meiosis and individualize in preparation for activation, morphogenesis, and the acquisition of motility. Finally, in addition to DNA, sperm contribute specific cellular factors that contribute to successful embryogenesis.
Figures



References
-
- Achanzar WE, Ward S (1997) A nematode gene required for sperm vesicle fusion. J Cell Sci 110(Pt 9):1073–1081 - PubMed
-
- Aitken RJ, De Iuliis GN (2007) Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online 14(6):727–733 - PubMed
-
- Albertson DG (1984) Formation of the first cleavage spindle in nematode embryos. Dev Biol 101(1):61–72 - PubMed
-
- Albertson DG, Thomson JN (1993) Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res 1(1):15–26 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

