Phase 1b dose-finding study of motesanib with docetaxel or paclitaxel in patients with metastatic breast cancer
- PMID: 22872523
- PMCID: PMC3413817
- DOI: 10.1007/s10549-012-2135-0
Phase 1b dose-finding study of motesanib with docetaxel or paclitaxel in patients with metastatic breast cancer
Abstract
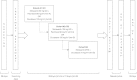
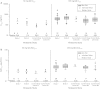
The purpose of this study was to investigate the safety, tolerability, and pharmacokinetics of motesanib when combined with docetaxel or paclitaxel in patients with metastatic breast cancer. In this open-label, dose-finding, phase 1b study, patients received motesanib 50 or 125-mg orally once daily (QD), beginning day 3 of cycle 1 of chemotherapy, continuously in combination with either paclitaxel 90 mg/m(2) on days 1, 8, and 15 every 28-day cycle (Arm A) or docetaxel 100 mg/m(2) on day 1 every 21-day cycle (Arm B). Dose escalation to motesanib 125 mg QD occurred if the incidence of dose-limiting toxicities (DLTs, primary endpoint) was ≤ 33 %. If the maximum tolerated dose (MTD) of motesanib was established in Arm B, additional patients could receive motesanib at the MTD plus docetaxel 75 mg/m(2). Forty-six patients were enrolled and 45 received ≥ 1 dose of motesanib. The incidence of DLTs was <33 % in all cohorts; thus, motesanib 125 mg QD was established as the MTD. Seven patients (16 %) had grade 3 motesanib-related adverse events including cholecystitis (2 patients) and hypertension (2 patients). Pharmacokinetic parameters of motesanib were similar to those reported in previous studies. The objective response rate was 56 % among patients with measurable disease at baseline who received motesanib in combination with taxane-based chemotherapy. The addition of motesanib to either paclitaxel or docetaxel was generally tolerable up to the 125-mg QD dose of motesanib. The objective response rate of 56 % suggests a potential benefit of motesanib in combination with taxane-based chemotherapy.
Figures


Similar articles
-
Phase 1b study of motesanib, an oral angiogenesis inhibitor, in combination with carboplatin/paclitaxel and/or panitumumab for the treatment of advanced non-small cell lung cancer.Clin Cancer Res. 2010 Jan 1;16(1):279-90. doi: 10.1158/1078-0432.CCR-09-1675. Epub 2009 Dec 22. Clin Cancer Res. 2010. PMID: 20028752 Clinical Trial.
-
Safety and pharmacokinetics of motesanib in combination with gemcitabine and erlotinib for the treatment of solid tumors: a phase 1b study.BMC Cancer. 2011 Jul 26;11:313. doi: 10.1186/1471-2407-11-313. BMC Cancer. 2011. PMID: 21791058 Free PMC article. Clinical Trial.
-
A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer.Ann Oncol. 2011 Sep;22(9):2057-2067. doi: 10.1093/annonc/mdq731. Epub 2011 Feb 14. Ann Oncol. 2011. PMID: 21321086 Clinical Trial.
-
Motesanib, or open-label bevacizumab, in combination with paclitaxel, as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a phase 2, randomised, double-blind, placebo-controlled study.Lancet Oncol. 2011 Apr;12(4):369-76. doi: 10.1016/S1470-2045(11)70037-7. Epub 2011 Mar 21. Lancet Oncol. 2011. PMID: 21429799 Clinical Trial.
-
Docetaxel in combination chemotherapy for metastatic breast cancer.Semin Oncol. 1997 Aug;24(4 Suppl 13):S13-19-S13-26. Semin Oncol. 1997. PMID: 9335513 Review.
Cited by
-
Treatment Considerations for the Management of Patients With Hormone Receptor-Positive Metastatic Breast Cancer.J Adv Pract Oncol. 2014 Sep-Oct;5(5):321-30. J Adv Pract Oncol. 2014. PMID: 26114012 Free PMC article. Review.
-
Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy.J Hematol Oncol. 2022 Jul 7;15(1):89. doi: 10.1186/s13045-022-01310-7. J Hematol Oncol. 2022. PMID: 35799213 Free PMC article. Review.
References
-
- Gordon MS, Mendelson DS, Kato G. Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer. 2009;126(8):1777–1787. - PubMed
-
- Foekens JA, Peters HA, Grebenchtchikov N, Look MP, Meijer-van Gelder ME, Geurts-Moespot A, van der Kwast TH, Sweep CG, Klijn JG. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61(14):5407–5414. - PubMed
-
- Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18(7):1423–1431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

