A novel core fucose-specific lectin from the mushroom Pholiota squarrosa
- PMID: 22872641
- PMCID: PMC3464508
- DOI: 10.1074/jbc.M111.327692
A novel core fucose-specific lectin from the mushroom Pholiota squarrosa
Abstract
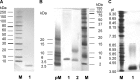
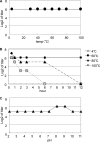
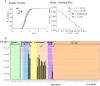
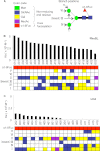
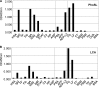
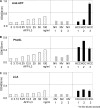
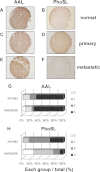
Fucα1-6 oligosaccharide has a variety of biological functions and serves as a biomarker for hepatocellular carcinoma because of the elevated presence of fucosylated α-fetoprotein (AFP) in this type of cancer. In this study we purified a novel Fucα1-6-specific lectin from the mushroom Pholiota squarrosa by ion-exchange chromatography and affinity chromatography on thyroglobulin-agarose. The purified lectin was designated as PhoSL (P. squarrosa lectin). SDS-PAGE, MALDI-TOF mass spectrometry, and N-terminal amino acid sequencing indicate that PhoSL has a molecular mass of 4.5 kDa and consists of 40 amino acids (NH(2)-APVPVTKLVCDGDTYKCTAYLDFGDGRWVAQWDTNVFHTG-OH). Isoelectric focusing of the lectin showed bands near pI 4.0. The lectin activity was stable between pH 2.0 and 11.0 and at temperatures ranging from 0 to 100 °C for incubation times of 30 min. When PhoSL was investigated with frontal affinity chromatography using 132 pyridylaminated oligosaccharides, it was found that the lectin binds only to core α1-6-fucosylated N-glycans and not to other types of fucosylated oligosaccharides, such as α1-2-, α1-3-, and α1-4-fucosylated glycans. Furthermore, PhoSL bound to α1-6-fucosylated AFP but not to non-fucosylated AFP. In addition, PhoSL was able to demonstrate the differential expression of α1-6 fucosylation between primary and metastatic colon cancer tissues. Thus, PhoSL will be a promising tool for analyzing the biological functions of α1-6 fucosylation and evaluating Fucα1-6 oligosaccharides as cancer biomarkers.
Figures
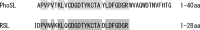
Similar articles
-
Structural basis for specific recognition of core fucosylation in N-glycans by Pholiota squarrosa lectin (PhoSL).Glycobiology. 2019 Jul 1;29(7):576-587. doi: 10.1093/glycob/cwz025. Glycobiology. 2019. PMID: 30913288
-
Core-fucose-specific Pholiota squarrosa lectin decreased hepatic inflammatory macrophage infiltration in steatohepatitis mice.Glycoconj J. 2024 Oct;41(4-5):267-278. doi: 10.1007/s10719-024-10163-w. Epub 2024 Sep 9. Glycoconj J. 2024. PMID: 39249179
-
Glycan array analysis of Pholiota squarrosa lectin and other fucose-oriented lectins.Glycobiology. 2021 May 3;31(4):459-476. doi: 10.1093/glycob/cwaa093. Glycobiology. 2021. PMID: 33021632 Free PMC article.
-
Lectin immunohistochemisty with Pholiota squarrosa lectin to evaluate core fucose.2021 Aug 24 [updated 2022 Apr 1]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2021 Aug 24 [updated 2022 Apr 1]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590764 Free Books & Documents. Review. No abstract available.
-
Fucosylated haptoglobin is a novel marker for pancreatic cancer: detailed analyses of oligosaccharide structures.Proteomics. 2008 Aug;8(16):3257-62. doi: 10.1002/pmic.200800046. Proteomics. 2008. PMID: 18646007 Review.
Cited by
-
Fucosylation is a common glycosylation type in pancreatic cancer stem cell-like phenotypes.World J Gastroenterol. 2015 Apr 7;21(13):3876-87. doi: 10.3748/wjg.v21.i13.3876. World J Gastroenterol. 2015. PMID: 25852272 Free PMC article.
-
Bisecting GlcNAc Is a General Suppressor of Terminal Modification of N-glycan.Mol Cell Proteomics. 2019 Oct;18(10):2044-2057. doi: 10.1074/mcp.RA119.001534. Epub 2019 Aug 2. Mol Cell Proteomics. 2019. PMID: 31375533 Free PMC article.
-
Suppression of tumor growth by Pleurotus ferulae ethanol extract through induction of cell apoptosis, and inhibition of cell proliferation and migration.PLoS One. 2014 Jul 16;9(7):e102673. doi: 10.1371/journal.pone.0102673. eCollection 2014. PLoS One. 2014. PMID: 25029345 Free PMC article.
-
Reevaluation of Pholiota squarrosa lectin-reactive haptoglobin as a pancreatic cancer biomarker using an improved ELISA system.Glycoconj J. 2017 Aug;34(4):537-544. doi: 10.1007/s10719-017-9772-9. Epub 2017 Apr 28. Glycoconj J. 2017. PMID: 28455724 Free PMC article.
-
Upregulation of glycans containing 3' fucose in a subset of pancreatic cancers uncovered using fusion-tagged lectins.J Proteome Res. 2015 Jun 5;14(6):2594-605. doi: 10.1021/acs.jproteome.5b00142. Epub 2015 May 12. J Proteome Res. 2015. PMID: 25938165 Free PMC article.
References
-
- Miyoshi E., Moriwaki K., Nakagawa T. (2008) Biological function of fucosylation in cancer biology. J. Biochem. 143, 725–729 - PubMed
-
- Uozumi N, Yanagidani S, Miyoshi E, Ihara Y, Sakuma T, Gao CX, Teshima T, Fujii S, Shiba T, Taniguchi N. (1996) Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-β-d-glucosaminide α1→6fucosyltransferase. J. Biol. Chem. 271, 27810–27817 - PubMed
-
- Tateno H., Nakamura-Tsuruta S., Hirabayashi J. (2009) Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 19, 527–536 - PubMed
-
- Howard I. K. (1971) Studies on a phytohemagglutinin from the lentil. II. Multiple forms of Lens culinaris hemagglutinin. J. Biol. Chem. 246, 1590–1595 - PubMed
-
- Foriers A., Lebrun E., Van Rapenbusch R., de Neve R., Strosberg A. D. (1981) The structure of the lentil (Lens culinaris) lectin. Amino acid sequence determination and prediction of the secondary structure. J. Biol. Chem. 256, 5550–5560 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

