Fibrils colocalize caspase-3 with procaspase-3 to foster maturation
- PMID: 22872644
- PMCID: PMC3460474
- DOI: 10.1074/jbc.M112.386128
Fibrils colocalize caspase-3 with procaspase-3 to foster maturation
Abstract
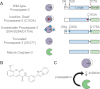
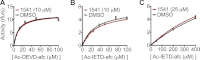
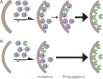
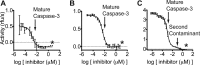
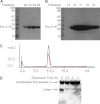
Most proteases are expressed as inactive precursors, or zymogens, that become activated by limited proteolysis. We previously identified a small molecule, termed 1541, that dramatically promotes the maturation of the zymogen, procaspase-3, to its mature form, caspase-3. Surprisingly, compound 1541 self-assembles into nanofibrils, and localization of procaspase-3 to the fibrils promotes activation. Here, we interrogate the biochemical mechanism of procaspase-3 activation on 1541 fibrils in addition to proteogenic amyloid-β(1-40) fibrils. In contrast to previous reports, we find no evidence that procaspase-3 alone is capable of self-activation, consistent with its fate-determining role in executing apoptosis. In fact, mature caspase-3 is >10(7)-fold more active than procaspase-3, making this proenzyme a remarkably inactive zymogen. However, we also show that fibril-induced colocalization of trace amounts of caspase-3 or other initiator proteases with procaspase-3 dramatically stimulates maturation of the proenzyme in vitro. Thus, similar to known cellular signaling complexes, these synthetic or natural fibrils can serve as platforms to concentrate procaspase-3 for trans-activation by upstream proteases.
Figures

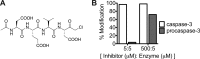



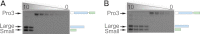

References
-
- Turk B. (2006) Targeting proteases. Successes, failures, and future prospects. Nat. Rev. Drug Discov. 5, 785–799 - PubMed
-
- Stroud R. M., Kossiakoff A. A., Chambers J. L. (1977) Mechanisms of zymogen activation. Annu. Rev. Biophys. Bioeng. 6, 177–193 - PubMed
-
- Donepudi M., Grütter M. G. (2002) Structure and zymogen activation of caspases. Biophys. Chem. 101, 145–153 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

