Sphingopeptides: dihydrosphingosine-based fusion inhibitors against wild-type and enfuvirtide-resistant HIV-1
- PMID: 22872679
- PMCID: PMC3475257
- DOI: 10.1096/fj.12-215111
Sphingopeptides: dihydrosphingosine-based fusion inhibitors against wild-type and enfuvirtide-resistant HIV-1
Abstract
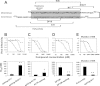
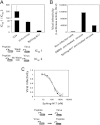
Understanding the structural organization of lipids in the cell and viral membranes is essential for elucidating mechanisms of viral fusion that lead to entry of enveloped viruses into their host cells. The HIV lipidome shows a remarkable enrichment in dihydrosphingomyelin, an unusual sphingolipid formed by a dihydrosphingosine backbone. Here we investigated the ability of dihydrosphingosine to incorporate into the site of membrane fusion mediated by the HIV envelope (Env) protein. Dihydrosphingosine as well as cholesterol, fatty acid, and tocopherol was conjugated to highly conserved, short HIV-1 Env-derived peptides with no antiviral activity otherwise. We showed that dihydrosphingosine exclusively endowed nanomolar antiviral activity to the peptides (IC(50) as low as 120 nM) in HIV-1 infection on TZM-bl cells and on Jurkat T cells, as well as in the cell-cell fusion assay. These sphingopeptides were active against enfuvirtide-resistant and wild-type CXCR4 and CCR5 tropic HIV strains. The anti-HIV activity was determined by both the peptides and their dihydrosphingosine conjugate. Moreover, their mode of action involved accumulation in the cells and viruses and binding to membranes enriched in sphingomyelin and cholesterol. The data suggest that sphingopeptides are recruited to the HIV membrane fusion site and provide a general concept in developing inhibitors of sphingolipid-mediated biological systems.
Figures



References
-
- White J. M. (1990) Viral and cellular membrane fusion proteins. Annu. Rev. Physiol. 52, 675–697 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

