Positron lymphography: multimodal, high-resolution, dynamic mapping and resection of lymph nodes after intradermal injection of 18F-FDG
- PMID: 22872741
- PMCID: PMC3537831
- DOI: 10.2967/jnumed.112.104349
Positron lymphography: multimodal, high-resolution, dynamic mapping and resection of lymph nodes after intradermal injection of 18F-FDG
Abstract
The lymphatic system plays a critical role in the maintenance of healthy tissues. Its function is an important indicator of the presence and extent of disease. In oncology, metastatic spread to local lymph nodes (LNs) is a strong predictor of poor outcome. Clinical methods for the visualization of LNs involve regional injection and tracking of (99m)Tc-sulfur colloid ((99m)Tc-SC) along with absorbent dyes. Intraoperatively, these techniques suffer from the requirement of administration of multiple contrast media ((99m)Tc-SC and isosulfan blue), unwieldy γ-probes, and a short effective surgical window for dyes. Preclinically, imaging of transport through the lymphatics is further hindered by the resolution of lymphoscintigraphy and SPECT. We investigated multimodal imaging in animal models using intradermal administration of (18)F-FDG for combined diagnostic and intraoperative use. PET visualizes LNs with high sensitivity and resolution and low background. Cerenkov radiation (CR) from (18)F-FDG was evaluated to optically guide surgical resection of LNs.
Methods: Imaging of (18)F-FDG uptake used PET and sensitive luminescent imaging equipment (for CR). Dynamic PET was performed in both sexes and multiple strains (NCr Nude, C57BL/6, and Nu/Nu) of mice. Biodistribution confirmed the uptake of (18)F-FDG and was compared with that of (99m)Tc-SC. Verification of uptake and the ability to use (18)F-FDG CR to guide nodal removal were confirmed histologically.
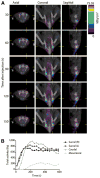
Results: Intradermal injection of (18)F-FDG clearly revealed lymphatic vessels and LNs by PET. Dynamic imaging revealed rapid and sustained labeling of these structures. Biodistribution of the radiotracer confirmed the active transport of radioglucose in the lymphatics to the local LNs and over time into the general circulation. (18)F-FDG also enabled visualization of LNs through CR, even before surgically revealing the site, and guided LN resection.
Conclusion: Intradermal (18)F-FDG can enhance the preclinical investigation of the lymphatics through dynamic, high-resolution, and quantitative tomographic imaging. Clinically, combined PET/Cerenkov imaging has significant potential as a single-dose, dual-modality tracer for diagnostics (PET/CT) and guided resection of LNs (Cerenkov optical).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures


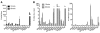
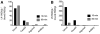

References
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. - PubMed
-
- Lucey BC, Stuhlfaut JW, Soto JA. Mesenteric lymph nodes seen at imaging: causes and significance. Radiographics. 2005;25:351–365. - PubMed
-
- Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer Staging System for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. - PubMed
-
- Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–983. - PubMed
-
- Pagliarulo V, Hawes D, Brands FH, et al. Detection of occult lymph node metastases in locally advanced node-negative prostate cancer. J Clin Oncol. 2006;24:2735–2742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
