Radiation-Triggered NF-κB Activation is Responsible for the Angiogenic Signaling Pathway and Neovascularization for Breast Cancer Cell Proliferation and Growth
- PMID: 22872788
- PMCID: PMC3411495
- DOI: 10.4137/BCBCR.S9592
Radiation-Triggered NF-κB Activation is Responsible for the Angiogenic Signaling Pathway and Neovascularization for Breast Cancer Cell Proliferation and Growth
Abstract
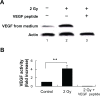
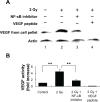
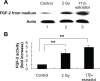
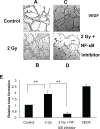
Tumors require blood supply to survive, grow, and metastasize. This involves the process of angiogenesis signaling for new blood vessel growth into a growing tumor mass. Understanding the mechanism of the angiogenic signaling pathway and neovascularization for breast cancer cell proliferation and growth would help to develop molecular interventions and achieve disease free survival. Our hypothesis is that the surviving cancer cell(s) after radiotherapy can initiate angiogenic signaling pathway in the neighboring endothelial cells resulting in neovascularization for breast cancer cell growth. The angiogenic signaling pathway is initiated by angiogenic factors, VEGF and FGF-2, through activation of a transcriptional regulator NF-κB, which in turn is triggered by therapeutic doses of radiation exposure Human breast adenocarcinoma cells (MCF-7 cells) were exposed to Cesium-137 ((137)Cs) γ rays to a total dose of 2 Gy at a dose rate of 1.03 Gy/min. The results of mobility shift assay showed that radiation at clinical doses (2 Gy) could induce NF-κB DNA-binding activity. Then, we examined the communication of angiogenic signals from irradiated MCF-7 cells to vascular endothelial cells. At the protein level, the western blot showed induction of angiogenic factors VEGF and FGF-2 in MCF-7 cells irradiated with 2 Gy. Inhibition of NF-κB activation attenuated VEGF and FGF-2 levels. These factors are secreted into the medium. The levels of VEGF and FGF-2 in the extra cellular medium were both increased, after 2 Gy exposures. We also observed corresponding expression of VEGFR2 and FGFR1 in non-irradiated endothelial cells that were co-cultured with irradiated MCF-7 cells. In support of this, in vitro tube formation assays provided evidence that irradiated MCF-7 cells transmit signals to potentiate cultured non-irradiated endothelial cells to form tube networks, which is the hallmark of neovascularization. Inhibition of NF-κB activation attenuated irradiated MCF-7-induced tube network formation. The data provide evidence that the radiation exposure is responsible for tumor growth and maintenance by inducing an angiogenic signaling pathway through activation of NF-κB.
Keywords: NF-κB activation; angiogenic factors; breast cancer; neovascularization.
Figures




Similar articles
-
Inter- and intra-cellular mechanism of NF-kB-dependent survival advantage and clonal expansion of radio-resistant cancer cells.Cell Signal. 2017 Feb;31:105-111. doi: 10.1016/j.cellsig.2017.01.011. Epub 2017 Jan 6. Cell Signal. 2017. PMID: 28069440
-
Bystander effects of ionizing radiation: conditioned media from X-ray irradiated MCF-7 cells increases the angiogenic ability of endothelial cells.Cell Commun Signal. 2019 Dec 16;17(1):165. doi: 10.1186/s12964-019-0474-8. Cell Commun Signal. 2019. PMID: 31842899 Free PMC article.
-
Effects of leptin-modified human placenta-derived mesenchymal stem cells on angiogenic potential and peripheral inflammation of human umbilical vein endothelial cells (HUVECs) after X-ray radiation.J Zhejiang Univ Sci B. 2020 Apr.;21(4):327-340. doi: 10.1631/jzus.B1900598. J Zhejiang Univ Sci B. 2020. PMID: 32253842 Free PMC article.
-
Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy.Curr Med Chem Anticancer Agents. 2003 Mar;3(2):95-117. doi: 10.2174/1568011033353452. Curr Med Chem Anticancer Agents. 2003. PMID: 12678905 Review.
-
NF-κB Signaling in Tumor Pathways Focusing on Breast and Ovarian Cancer.Oncol Rev. 2022 Oct 3;16:10568. doi: 10.3389/or.2022.10568. eCollection 2022. Oncol Rev. 2022. PMID: 36531159 Free PMC article. Review.
Cited by
-
Combining antiangiogenic therapy and radiation in nasopharyngeal carcinoma.Saudi Med J. 2015 Jun;36(6):659-64. doi: 10.15537/smj.2015.6.11460. Saudi Med J. 2015. PMID: 25987106 Free PMC article. Review.
-
Down-regulation of platelet-derived growth factor-D expression blockades NF-κB pathway to inhibit cell proliferation and invasion as well as induce apoptosis in esophageal squamous cell carcinoma.Mol Biol Rep. 2013 Mar;40(3):2473-83. doi: 10.1007/s11033-012-2328-y. Epub 2012 Nov 28. Mol Biol Rep. 2013. PMID: 23187740
-
Interference with pathways activated by topoisomerase inhibition alters the surface expression of PD-L1 and MHC I in colon cancer cells.Oncol Lett. 2022 Dec 9;25(1):41. doi: 10.3892/ol.2022.13628. eCollection 2023 Jan. Oncol Lett. 2022. PMID: 36589674 Free PMC article.
-
Combing MRI Perfusion and 18F-FDG PET/CT Metabolic Biomarkers Helps Predict Survival in Advanced Nasopharyngeal Carcinoma: A Prospective Multimodal Imaging Study.Cancers (Basel). 2021 Mar 28;13(7):1550. doi: 10.3390/cancers13071550. Cancers (Basel). 2021. PMID: 33800542 Free PMC article.
-
COX-2 promotes breast cancer cell radioresistance via p38/MAPK-mediated cellular anti-apoptosis and invasiveness.Tumour Biol. 2013 Oct;34(5):2817-26. doi: 10.1007/s13277-013-0840-x. Epub 2013 Jun 15. Tumour Biol. 2013. PMID: 23771849
References
-
- Foekens JA, Peters HA, Grebenchtchikov N, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–14. - PubMed
-
- Gasparini G, Toi M, Gion M, et al. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst. 1997;89:139–47. - PubMed
-
- Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–16. - PubMed
-
- Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18:1423–31. - PubMed
-
- Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8:483–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

