Lack of efficacy of a herbal preparation (RCM-102) for seasonal allergic rhinitis: a double blind, randomised, placebo-controlled trial
- PMID: 22872821
- PMCID: PMC3406298
- DOI: 10.5415/apallergy.2012.2.3.187
Lack of efficacy of a herbal preparation (RCM-102) for seasonal allergic rhinitis: a double blind, randomised, placebo-controlled trial
Abstract
Background: A herbal preparation, known as RMIT Chinese Medicine 102 (RCM-102) consisting of eight herbs which demonstrates inhibition of the release of key inflammatory mediators associated with seasonal allergic rhinitis (SAR) was used. This study evaluated the efficacy and safety of RCM-102 for SAR.
Objective: This study evaluated the efficacy and safety of RCM-102 for SAR.
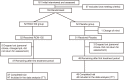
Methods: This randomised placebo-controlled trial involved subjects aged between 18 and 65 who were randomly assigned to either RCM-102 or a placebo group. After a two-week baseline period, all subjects took either RCM-102 or placebo capsules (two capsules each time, three times daily with a four hour interval) for a period of eight weeks. The primary end-points were the Five-Point Scale symptom scores. Rhinoconjunctivitis Quality of Life Questionnaire, relief medication usage, adverse events, kidney and liver function tests and full blood examination were secondary end-points. Intention-to-treat analysis was applied.
Results: One hundred and four subjects were randomised with 52 in each group. Ninety-five subjects (47 and 48 subjects in RCM-102 and placebo groups) completed the trial. Nine subjects withdrew from the study prior to the end of the second treatment week. At the end of the trial, there were no significant differences between the two groups with respect to all outcome measures. There were no liver or kidney function abnormalities reported.
Conclusion: This mechanism-based RCM-102 was safe but not more beneficial than placebo for patients with SAR.
Keywords: Herbal medicine; RCM-102; Seasonal allergic rhinitis.
Figures

Similar articles
-
Treatment for seasonal allergic rhinitis by Chinese herbal medicine: a randomized placebo controlled trial.Altern Ther Health Med. 2003 Sep-Oct;9(5):80-7. Altern Ther Health Med. 2003. PMID: 14526714 Clinical Trial.
-
Effect of adding a Chinese herbal preparation to acupuncture for seasonal allergic rhinitis: randomised double-blind controlled trial.Hong Kong Med J. 2003 Dec;9(6):427-34. Hong Kong Med J. 2003. PMID: 14660810 Clinical Trial.
-
Efficacy and Safety of a Chinese Herbal Medicine Formula (RCM-104) in the Management of Simple Obesity: A Randomized, Placebo-Controlled Clinical Trial.Evid Based Complement Alternat Med. 2012;2012:435702. doi: 10.1155/2012/435702. Epub 2012 Feb 20. Evid Based Complement Alternat Med. 2012. PMID: 22550541 Free PMC article.
-
Levocetirizine for the treatment of allergic rhinitis and chronic idiopathic urticaria in adults and children.Clin Ther. 2009 Aug;31(8):1664-87. doi: 10.1016/j.clinthera.2009.08.015. Clin Ther. 2009. PMID: 19808127 Review.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
Cited by
-
The beliefs about allergic rhinitis and its treatment options from people in Central Thailand.Asia Pac Allergy. 2022 Jan 26;12(1):e11. doi: 10.5415/apallergy.2022.12.e11. eCollection 2022 Jan. Asia Pac Allergy. 2022. PMID: 35174062 Free PMC article.
-
Herbal Medicines for Allergic Rhinitis: a Systematic Review and Meta-analysis.Curr Allergy Asthma Rep. 2021 Mar 25;21(4):25. doi: 10.1007/s11882-021-00999-9. Curr Allergy Asthma Rep. 2021. PMID: 33768322
-
Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis.Allergy Asthma Immunol Res. 2018 Jul;10(4):300-353. doi: 10.4168/aair.2018.10.4.300. Allergy Asthma Immunol Res. 2018. PMID: 29949830 Free PMC article. Review.
-
Chinese Herbal Medicine to Treat Allergic Rhinitis: Evidence From a Meta-Analysis.Allergy Asthma Immunol Res. 2018 Jan;10(1):34-42. doi: 10.4168/aair.2018.10.1.34. Allergy Asthma Immunol Res. 2018. PMID: 29178676 Free PMC article.
-
Complementary and alternative therapy (CAM) in the treatment of allergic rhinitis.Curr Allergy Asthma Rep. 2014 Dec;14(12):479. doi: 10.1007/s11882-014-0479-8. Curr Allergy Asthma Rep. 2014. PMID: 25269403 Review.
References
-
- Xue CC, Hügel HM, Li CG, Story DF. Efficacy, chemistry and pharmacology of Chinese herbal medicine for allergic rhinitis. Curr Med Chem. 2004;11:1403–1421. - PubMed
-
- Xue CC, Li CG, Hügel HM, Story DF. Does acupuncture or Chinese herbal medicine have a role in the treatment of allergic rhinitis? Curr Opin Allergy Clin Immunol. 2006;6:175–179. - PubMed
-
- Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther. 2000;86:191–198. - PubMed
-
- Wang M, Lamers RJ, Korthout HA, van Nesselrooij JH, Witkamp RF, van der Heijden R, Voshol PJ, Havekes LM, Verpoorte R, van der Greef J. Metabolomics in the context of systems biology: bridging traditional Chinese medicine and molecular pharmacology. Phytother Res. 2005;19:173–182. - PubMed
-
- Tang JL, Leung PC. An efficacy-driven approach to the research and development of traditional Chinese medicine. Hong Kong Med J. 2001;7:375–380. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
