Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics
- PMID: 22872860
- PMCID: PMC3443140
- DOI: 10.1073/pnas.1211138109
Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics
Abstract
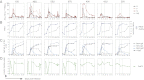
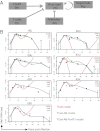
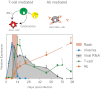
Measles virus (MeV) is the poster child for acute infection followed by lifelong immunity. However, recent work shows the presence of MeV RNA in multiple sites for up to 3 mo after infection in a proportion of infected children. Here, we use experimental infection of rhesus macaques to show that prolonged RNA presence is characteristic of primary infection. We found that viral RNA persisted in the blood, respiratory tract, or lymph nodes four to five times longer than the infectious virus and that the clearance of MeV RNA from blood happened in three phases: rapid decline coincident with clearance of infectious virus, a rebound phase with increases up to 10-fold, and a phase of slow decrease to undetectable levels. To examine the effect of individual host immune factors on MeV load dynamics further, we developed a mathematical model that expressed viral replication and elimination in terms of the strength of MeV-specific T-cell responses, antibody responses, target cell limitations, and immunosuppressive activity of regulatory T cells. Based on the model, we demonstrate that viral dynamics, although initially regulated by T cells, require antibody to eliminate viral RNA. These results have profound consequences for our view of acute viral infections, the development of prolonged immunity, and, potentially, viral evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Measles immunometrics.Proc Natl Acad Sci U S A. 2012 Sep 11;109(37):14724-5. doi: 10.1073/pnas.1212243109. Epub 2012 Aug 30. Proc Natl Acad Sci U S A. 2012. PMID: 22936050 Free PMC article. No abstract available.
References
-
- Simons E, et al. Assessment of the 2010 global measles mortality reduction goal: Results from a model of surveillance data. Lancet. 2012;379:2173–2178. - PubMed
-
- Panum P. Observations made during the epidemic of measles on the Faroe Islands in the year 1846. Med Classics. 1938;3:829–886.
-
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford Science Publications; 1992.
-
- Bjornstad ON. Dynamics of measles epidemics: Estimating scaling of transmission rates using a time series SIR model. Ecol Monogr. 2002;72(2):169–184.
-
- Grenfell BT. Dynamics of measles epidemics: Scaling noise, determinism, and predictability with the TSIR model. Ecol Monogr. 2002;72(2):185–202.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

