Molecular dynamics simulations on aqueous two-phase systems - Single PEG-molecules in solution
- PMID: 22873343
- PMCID: PMC3469337
- DOI: 10.1186/2046-1682-5-14
Molecular dynamics simulations on aqueous two-phase systems - Single PEG-molecules in solution
Abstract
Background: Molecular Dynamics (MD) simulations are a promising tool to generate molecular understanding of processes related to the purification of proteins. Polyethylene glycols (PEG) of various length are commonly used in the production and purification of proteins. The molecular mechanisms behind PEG driven precipitation, aqueous two-phase formation or the effects of PEGylation are however still poorly understood.
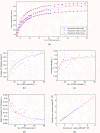
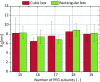
Results: In this paper, we ran MD simulations of single PEG molecules of variable length in explicitly simulated water. The resulting structures are in good agreement with experimentally determined 3D structures of PEG. The increase in surface hydrophobicity of PEG of longer chain length could be explained on an atomic scale. PEG-water interactions as well as aqueous two-phase formation in the presence of PO4 were found to be correlated to PEG surface hydrophobicity.
Conclusions: We were able to show that the taken MD simulation approach is capable of generating both structural data as well as molecule descriptors in agreement with experimental data. Thus, we are confident of having a good in silico representation of PEG.
Figures






References
-
- Atha DH, Ingham KC. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J biol Chem. 1981;256(2323):12108–17. - PubMed
-
- Albertsson PÅ. Particle fractionation in liquid two-phase systems The composition of some phase systems and the behavior of some model particles in them application to the isolation of cell walls from microorganisms. Biochim Biophys Acta. 1958;27:378–395. - PubMed
-
- Hansson UB, Wingren C. Separation of antibodies by liquid-liquid aqueous partition and by liquid-liquid partition chromatography. Sep Purif Methods. 1998;27(2):169–211. doi: 10.1080/03602549809351640. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

