Structural/functional analysis of the human OXR1 protein: identification of exon 8 as the anti-oxidant encoding function
- PMID: 22873401
- PMCID: PMC3462732
- DOI: 10.1186/1471-2199-13-26
Structural/functional analysis of the human OXR1 protein: identification of exon 8 as the anti-oxidant encoding function
Abstract
Background: The human OXR1 gene belongs to a class of genes with conserved functions that protect cells from reactive oxygen species (ROS). The gene was found using a screen of a human cDNA library by its ability to suppress the spontaneous mutator phenotype of an E. coli mutH nth strain. The function of OXR1 is unknown. The human and yeast genes are induced by oxidative stress and targeted to the mitochondria; the yeast gene is required for resistance to hydrogen peroxide. Multiple spliced isoforms are expressed in a variety of human tissues, including brain.
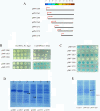
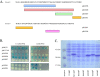
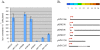
Results: In this report, we use a papillation assay that measures spontaneous mutagenesis of an E. coli mutM mutY strain, a host defective for oxidative DNA repair. Papillation frequencies with this strain are dependent upon a G→T transversion in the lacZ gene (a mutation known to occur as a result of oxidative damage) and are suppressed by in vivo expression of human OXR1. N-terminal, C-terminal and internal deletions of the OXR1 gene were constructed and tested for suppression of the mutagenic phenotype of the mutM mutY strain. We find that the TLDc domain, encoded by the final four exons of the OXR1 gene, is not required for papillation suppression in E. coli. Instead, we show that the protein segment encoded by exon 8 of OXR1 is responsible for the suppression of oxidative damage in E. coli.
Conclusion: The protein segment encoded by OXR1 exon 8 plays an important role in the anti-oxidative function of the human OXR1 protein. This result suggests that the TLDc domain, found in OXR1 exons 12-16 and common in many proteins with nuclear function, has an alternate (undefined) role other than oxidative repair.
Figures




References
-
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29(Pt 2):345–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

