Influence of two different levels of intra-abdominal hypertension on bacterial translocation in a porcine model
- PMID: 22873417
- PMCID: PMC3390291
- DOI: 10.1186/2110-5820-2-S1-S17
Influence of two different levels of intra-abdominal hypertension on bacterial translocation in a porcine model
Abstract
Background: The purpose of the present study was to quantify bacterial translocation to mesenteric lymph nodes due to different levels of intra-abdominal hypertension (IAH; 15 vs. 30 mmHg) lasting for 24 h in a porcine model.
Methods: We examined 18 anesthetized and intubated pigs (52.3 ± 4.7 kg) which were randomly allocated to three experimental groups (each n = 6) and studied over a period of 24 h. After preparation and establishing a steady state, the intra-abdominal pressure (IAP) was increased stepwise to 30 mmHg in six animals using a carbon dioxide (CO2) insufflator (IAP-30 group). In the second group, IAP was increased to 15 mmHg (IAP-15 group), while IAP remained unchanged in another six pigs (control group). Using a pulse contour cardiac output (PiCCO®) monitoring system, hemodynamic parameters as well as blood gases were recorded periodically. Moreover, peripheral and portal vein blood samples were taken for microbiological examinations. Lymph nodes from the ileocecal junction were sampled during an intra-vital laparotomy at the end of the observational period. After sacrificing the animals, bowel tissue samples and corresponding mesenteric lymph nodes (MLN) were extracted for histopathological and microbiological analyses.
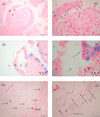
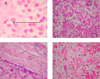
Results: Cardiac output decreased in all groups. In IAP-30 animals, volumetric preload indices significantly decreased, while those of IAP-15 pigs did not differ from those of controls. Under IAH, the mean arterial pressure (MAP) in the IAP-30 group declined, while MAP in the IAP-15 group was significantly elevated (controls unchanged). PO2 and PCO2 remained unchanged. The grade of ischemic damage of the intestines (histopathologically quantified using the Park score) increased significantly with different IAH levels. Accordingly, the amount of translocated bacteria in intestinal wall specimens as well as in MLN significantly increased with the level of IAH. Lymph node cultures confirmed the relation between bacterial translocation (BT) and IAP. The most often cultivated species were Escherichia coli, Staphylococcus, Clostridium, Pasteurella, and Streptococcus. Bacteremia was detected only occasionally in all three groups (not significantly different) showing gut-derived bacteria such as Proteus, Klebsiella, and E. coli spp.
Conclusion: In this porcine model, a higher level of ischemic damage and more BT were observed in animals subjected to an IAP of 30 mmHg when compared to animals subjected to an IAP of 15 mmHg or controls.
Figures


Similar articles
-
The effects of hemodynamic shock and increased intra-abdominal pressure on bacterial translocation.J Trauma. 2002 Jan;52(1):13-7. doi: 10.1097/00005373-200201000-00005. J Trauma. 2002. PMID: 11791046
-
Increased intra-abdominal pressure causes bacterial translocation in rabbits.J Chin Med Assoc. 2005 Apr;68(4):172-7. doi: 10.1016/S1726-4901(09)70244-8. J Chin Med Assoc. 2005. PMID: 15850067
-
[Influence of intra-abdominal hypertension on the intestinal permeability and endotoxin/bacteria translocation in rabbits].Zhonghua Shao Shang Za Zhi. 2003 Aug;19(4):229-32. Zhonghua Shao Shang Za Zhi. 2003. PMID: 14514405 Chinese.
-
What every ICU clinician needs to know about the cardiovascular effects caused by abdominal hypertension.Anaesthesiol Intensive Ther. 2015;47(4):388-99. doi: 10.5603/AIT.a2015.0028. Epub 2015 May 14. Anaesthesiol Intensive Ther. 2015. PMID: 25973663 Review.
-
[The patient with intra-abdominal hypertension].Anasthesiol Intensivmed Notfallmed Schmerzther. 2016 Jan;51(1):8-16. doi: 10.1055/s-0041-103160. Epub 2016 Feb 10. Anasthesiol Intensivmed Notfallmed Schmerzther. 2016. PMID: 26863642 Review. German.
Cited by
-
Unexpected Abscess Localization of the Anterior Abdominal Wall in an ADPKD Patient Undergoing Hemodialysis.Case Rep Nephrol. 2015;2015:982575. doi: 10.1155/2015/982575. Epub 2015 Aug 2. Case Rep Nephrol. 2015. PMID: 26301109 Free PMC article.
-
Time-course evaluation of intestinal structural disorders in a porcine model of intra-abdominal hypertension by mechanical intestinal obstruction.PLoS One. 2018 Jan 22;13(1):e0191420. doi: 10.1371/journal.pone.0191420. eCollection 2018. PLoS One. 2018. PMID: 29357386 Free PMC article.
-
MICU1 may be a promising intervention target for gut-derived sepsis induced by intra-abdominal hypertension.Cell Death Discov. 2016 Nov 28;2:16080. doi: 10.1038/cddiscovery.2016.80. eCollection 2016. Cell Death Discov. 2016. PMID: 27924224 Free PMC article.
-
Gut failure in critical care: old school versus new school.Ann Gastroenterol. 2015 Jul-Sep;28(3):309-322. Ann Gastroenterol. 2015. PMID: 26130136 Free PMC article. Review.
-
Effect of acute, slightly increased intra-abdominal pressure on intestinal permeability and oxidative stress in a rat model.PLoS One. 2014 Oct 8;9(10):e109350. doi: 10.1371/journal.pone.0109350. eCollection 2014. PLoS One. 2014. PMID: 25295715 Free PMC article.
References
-
- Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D'Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–1732. doi: 10.1007/s00134-006-0349-5. - DOI - PubMed
-
- Barnes GE, Laine GA, Giam PY, Smith EE, Granger HJ. Cardiovascular responses to elevation of intra-abdominal hydrostatic pressure. Am J Physiol. 1985;248:R208–13. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

