Wnt1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern β-amyloid apoptotic injury of microglia
- PMID: 22873724
- PMCID: PMC3521866
- DOI: 10.2174/156720212803530618
Wnt1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern β-amyloid apoptotic injury of microglia
Abstract
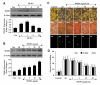
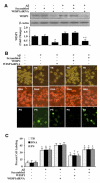
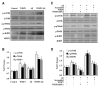
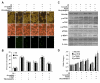
Given the present challenges to attain effective treatment for β-amyloid (Aβ) toxicity in neurodegenerative disorders such as Alzheimer's disease, development of novel cytoprotective pathways that can assist immune mediated therapies through the preservation of central nervous system microglia could offer significant promise. We show that the CCN4 protein, Wnt1 inducible signaling pathway protein 1 (WISP1), is initially up-regulated by Aβ and can modulate its endogenous expression for the protection of microglia during Aβ mediated apoptosis. WISP1 activates mTOR and phosphorylates p70S6K and 4EBP1 through the control of the regulatory mTOR component PRAS40. Loss of PRAS40 through gene reduction or inhibition by WISP1 is cytoprotective. WISP1 ultimately governs PRAS40 by sequestering PRAS40 intracellularly through post-translational phosphorylation and binding to protein 14-3-3. Our work identifies WISP1, mTOR signaling, and PRAS40 as targets for new strategies directed against Alzheimer's disease and related disorders.
Figures
References
-
- Macsai CE, Georgiou KR, Foster BK, Zannettino AC, Xian CJ. Microarray expression analysis of genes and pathways involved in growth plate cartilage injury responses and bony repair. Bone. 2012 May;50(5):1081–91. - PubMed
-
- Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, et al. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1839–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
