Evaluation of the effects of botulinum toxin A injections when used to improve ease of care and comfort in children with cerebral palsy whom are non-ambulant: a double blind randomized controlled trial
- PMID: 22873758
- PMCID: PMC3472230
- DOI: 10.1186/1471-2431-12-120
Evaluation of the effects of botulinum toxin A injections when used to improve ease of care and comfort in children with cerebral palsy whom are non-ambulant: a double blind randomized controlled trial
Abstract
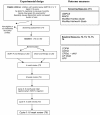
Background: Children with cerebral palsy (CP) whom are non-ambulant are at risk of reduced quality of life and poor health status. Severe spasticity leads to discomfort and pain. Carer burden for families is significant. This study aims to determine whether intramuscular injections of botulinum toxin A (BoNT-A) combined with a regime of standard therapy has a positive effect on care and comfort for children with CP whom are non-ambulant (GMFCS IV/V), compared with standard therapy alone (cycle I), and whether repeated injections with the same regime of adjunctive therapy results in greater benefits compared with a single injecting episode (cycle II). The regime of therapy will include serial casting, splinting and/or provision of orthoses, as indicated, combined with four sessions of goal directed occupational therapy or physiotherapy.
Method/design: This study is a double blind randomized controlled trial. Forty participants will be recruited. In cycle I, participants will be randomized to either a treatment group who will receive BoNT-A injections into selected upper and/or lower limb muscles, or a control group who will undergo sham injections. Both groups will receive occupational therapy and /or physiotherapy following injections. Groups will be assessed at baseline then compared at 4 and 16 weeks following injections or sham control. Parents, treating clinicians and assessors will be masked to group allocation. In cycle II, all participants will undergo intramuscular BoNT-A injections to selected upper and/or lower limb muscles, followed by therapy.The primary outcome measure will be change in parent ratings in identified areas of concern for their child's care and comfort, using the Canadian Occupational Performance Measure (COPM). Secondary measures will include the Care and Comfort Hypertonicity Scale (ease of care), the Cerebral Palsy Quality of Life Questionnaire (CP QoL-Child) (quality of life), the Caregiver Priorities and Child Health Index of Life with Disabilities Questionnaire (CPCHILD©) (health status) and the Paediatric Pain Profile (PPP) (pain). Adverse events will be carefully monitored by a clinician masked to group allocation.
Discussion: This paper outlines the theoretical basis, study hypotheses and outcome measures for a trial of BoNT-A injections and therapy for children with non-ambulant CP.
Trial registration: Australia New Zealand Clinical Trials Registry:N12609000360213.
Figures
References
-
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. - PubMed
-
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. - PubMed
-
- Howard J, Soo B, Graham HK, Boyd RN, Reid S, Lanigan A, Wolfe R, Reddihough DS. Cerebral palsy in Victoria: motor types, topography and gross motor function. J Paediatr Child Health. 2005;41(9–10):479–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

