Enhanced photoluminescence of porous silicon nanoparticles coated by bioresorbable polymers
- PMID: 22873790
- PMCID: PMC3464699
- DOI: 10.1186/1556-276X-7-446
Enhanced photoluminescence of porous silicon nanoparticles coated by bioresorbable polymers
Abstract
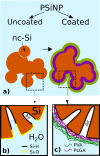
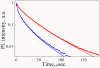
A significant enhancement of the photoluminescence (PL) efficiency is observed for aqueous suspensions of porous silicon nanoparticles (PSiNPs) coated by bioresorbable polymers, i.e., polylactic-co-glycolic acid (PLGA) and polyvinyl alcohol (PVA). PSiNPs with average size about 100 nm prepared by mechanical grinding of electrochemically etched porous silicon were dispersed in water to prepare the stable suspension. The inner hydrophobic PLGA layer prevents the PSiNPs from the dissolution in water, while the outer PVA layer makes the PSiNPs hydrophilic. The PL quantum yield of PLGA/PVA-coated PSiNPs was found to increase by three times for 2 weeks of the storage in water. The observed effect is explained by taking into account both suppression of the dissolution of PSiNPs in water and a process of the passivation of nonradiative defects in PSiNPs. The obtained results are interesting in view of the potential applications of PSiNPs in bioimaging.
Figures

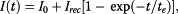
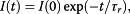
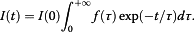
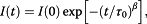
Similar articles
-
Recent advances in hybrid system of porous silicon nanoparticles and biocompatible polymers for biomedical applications.Biomed Eng Lett. 2021 Jun 15;11(3):171-181. doi: 10.1007/s13534-021-00194-9. eCollection 2021 Aug. Biomed Eng Lett. 2021. PMID: 34350046 Free PMC article. Review.
-
Amphiphilic Photoluminescent Porous Silicon Nanoparticles as Effective Agents for Ultrasound-Amplified Cancer Therapy.ACS Appl Mater Interfaces. 2025 Jan 8;17(1):374-385. doi: 10.1021/acsami.4c15725. Epub 2024 Dec 19. ACS Appl Mater Interfaces. 2025. PMID: 39701827
-
Formation of Si/SiO2 Luminescent Quantum Dots From Mesoporous Silicon by Sodium Tetraborate/Citric Acid Oxidation Treatment.Front Chem. 2019 Mar 29;7:165. doi: 10.3389/fchem.2019.00165. eCollection 2019. Front Chem. 2019. PMID: 30984738 Free PMC article.
-
Iron-silicate-coated porous silicon nanoparticles for in situ ROS self-generation.Colloids Surf B Biointerfaces. 2023 May;225:113273. doi: 10.1016/j.colsurfb.2023.113273. Epub 2023 Mar 22. Colloids Surf B Biointerfaces. 2023. PMID: 36965332
-
Photoluminescent and biodegradable porous silicon nanoparticles for biomedical imaging.J Mater Chem B. 2019 Nov 7;7(41):6271-6292. doi: 10.1039/c9tb01042d. Epub 2019 Aug 8. J Mater Chem B. 2019. PMID: 31393507 Review.
Cited by
-
Recent advances in hybrid system of porous silicon nanoparticles and biocompatible polymers for biomedical applications.Biomed Eng Lett. 2021 Jun 15;11(3):171-181. doi: 10.1007/s13534-021-00194-9. eCollection 2021 Aug. Biomed Eng Lett. 2021. PMID: 34350046 Free PMC article. Review.
References
-
- Poellinger A, Burock S, Grosenick D, Hagen A, Ludemann L, Diekmann F, Engelken F, Macdonald R, Rinneberg H, Schlag PM. Breast cancer: early- and late-fluorescence near-infrared imaging with indocyanine green – a preliminary study. Radiology. 2011;258:409–416. - PubMed
-
- Lim YT, Noh YW, Han JH, Cai QY, Yoon KH, Chung BH. Biocompatible polymer-nanoparticle-based bimodal imaging contrast agents for the labeling and tracking of dendritic cells. Small. 2008;10:1640–1645. - PubMed
-
- Grama CN, Ankola DD, Ravi Kumar MNV. Poly(lactide-co-glycolide) nanoparticles for peroral delivery of bioactives. Curr Op Coll & Interface Sci. 2011;16:238–245.
LinkOut - more resources
Full Text Sources
Miscellaneous

