Adhesion of mussel foot protein Mefp-5 to mica: an underwater superglue
- PMID: 22873939
- PMCID: PMC3428132
- DOI: 10.1021/bi3002538
Adhesion of mussel foot protein Mefp-5 to mica: an underwater superglue
Abstract
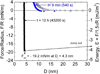
Mussels have a remarkable ability to attach their holdfast, or byssus, opportunistically to a variety of substrata that are wet, saline, corroded, and/or fouled by biofilms. Mytilus edulis foot protein-5 (Mefp-5) is one of several proteins in the byssal adhesive plaque of the mussel M. edulis. The high content of 3,4-dihydroxyphenylalanine (Dopa) (~30 mol %) and its localization near the plaque-substrate interface have often prompted speculation that Mefp-5 plays a key role in adhesion. Using the surface forces apparatus, we show that on mica surfaces Mefp-5 achieves an adhesion energy approaching E(ad) = ~-14 mJ/m(2). This exceeds the adhesion energy of another interfacial protein, Mefp-3, by a factor of 4-5 and is greater than the adhesion between highly oriented monolayers of biotin and streptavidin. The adhesion to mica is notable for its dependence on Dopa, which is most stable under reducing conditions and acidic pH. Mefp-5 also exhibits strong protein-protein interactions with itself as well as with Mefp-3 from M. edulis.
Figures








References
-
- Rubin DJ, Miserez A, Waite JH. Diverse strategies of protein sclerotization in marine invertebrate structure–property relationships in natural biomaterials. Adv. Insect Physiol. 2010;38:75–133.
-
- Wang J, Tahir MN, Kappl M, Tremel W, Metz N, Barz M, Theato P, Butt HJ. Influence of binding-site density in wet bioadhesion. Adv. Mater. 2008;20:3872–3876.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

