The hierarchical structure and mechanics of plant materials
- PMID: 22874093
- PMCID: PMC3479918
- DOI: 10.1098/rsif.2012.0341
The hierarchical structure and mechanics of plant materials
Abstract
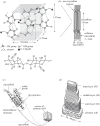
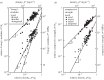
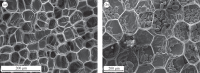
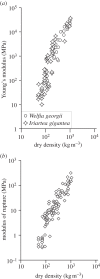
The cell walls in plants are made up of just four basic building blocks: cellulose (the main structural fibre of the plant kingdom) hemicellulose, lignin and pectin. Although the microstructure of plant cell walls varies in different types of plants, broadly speaking, cellulose fibres reinforce a matrix of hemicellulose and either pectin or lignin. The cellular structure of plants varies too, from the largely honeycomb-like cells of wood to the closed-cell, liquid-filled foam-like parenchyma cells of apples and potatoes and to composites of these two cellular structures, as in arborescent palm stems. The arrangement of the four basic building blocks in plant cell walls and the variations in cellular structure give rise to a remarkably wide range of mechanical properties: Young's modulus varies from 0.3 MPa in parenchyma to 30 GPa in the densest palm, while the compressive strength varies from 0.3 MPa in parenchyma to over 300 MPa in dense palm. The moduli and compressive strength of plant materials span this entire range. This study reviews the composition and microstructure of the cell wall as well as the cellular structure in three plant materials (wood, parenchyma and arborescent palm stems) to explain the wide range in mechanical properties in plants as well as their remarkable mechanical efficiency.
Figures








References
-
- Gibson L. J., Ashby M. F., Harley B. A. 2010. Cellular materials in nature and medicine. Cambridge, UK: Cambridge University Press
-
- Dinwoodie J. M. 1981. Timber: its nature and behaviour. New York, NY: Van Nostrand Reinhold
-
- Niklas K. J. 1992. Plant biomechanics. Chicago, IL: University of Chicago Press
-
- Hori R., Muller M., Watanabe U., Lichtenegger H. C., Fratzl P., Sugiyama J. 2002. The importance of seasonal differences in the cellulose microfibril angle in softwoods in determining acoustic properties. J. Mater. Sci. 37, 4279–428410.1023/A:1020688132345 (doi:10.1023/A:1020688132345) - DOI - DOI
-
- Peura M., Muller M., Vainio U., Saren M.-P., Saranpaa P., Serimaa R. 2008. X-ray microdiffraction reveals the orientation of cellulose microfibrils and the size of cellulose crystallites in single Norway spruce tracheids. Trees 22, 49–6110.1007/s00468-007-0168-5 (doi:10.1007/s00468-007-0168-5) - DOI - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
