IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal
- PMID: 22874466
- PMCID: PMC3810165
- DOI: 10.1161/CIRCRESAHA.112.270314
IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal
Abstract
Rationale: A high-fat diet accompanied by hypertriglyceridemia increases an individual's risk for development of atherosclerosis. An early event in this process is monocyte recruitment through binding to vascular cell adhesion molecule 1 (VCAM-1) upregulated on inflamed arterial endothelium. Diets high in polyunsaturated fatty acids (PUFAs) may provide athero-protection by ameliorating this effect.
Objective: We investigated the acute regulation of VCAM-1 expression in human aortic endothelial cells (HAEC) in response to triglyceride-rich lipoproteins (TGRL) isolated from subjects after consumption of a high-fat meal.
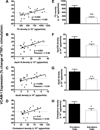
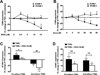
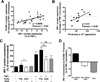
Methods and results: Postprandial TGRL isolated from 38 subjects were categorized as proatherogenic or antiatherogenic according to their capacity to alter the inflammatory response of HAEC. Proatherogenic TGRL increased expression of VCAM-1, intercellular adhesion molecule 1 (ICAM-1), and E-selectin by ≈20% compared with stimulation with tumor necrosis factor-α alone, whereas antiatherogenic TGRL decreased VCAM-1 expression by ≈20% while still upregulating ICAM-1. The relative atherogenicity of TGRL positively correlated with particle density of TG, apolipoprotein (Apo)CIII, ApoE, and cholesterol. Ω3-PUFA mimicked the effect of antiatherogenic TGRL by downregulating VCAM-1 expression. TGRL exerted this differential regulation of VCAM-1 by reciprocally modulating expression and activity of the transcription factor interferon regulatory factor 1 (IRF-1) and expression of microRNA 126 (miR-126). Overexpression or silencing of IRF-1 or miR-126 expression recapitulated the proatherogenic or antiatherogenic regulation of VCAM-1.
Conclusions: In response to a high-fat meal, TGRL bias the inflammatory response of endothelium via transcriptional and posttranscriptional editing of VCAM-1. Subjects with an anti-inflammatory response to a meal produced TGRL that was enriched in nonesterified fatty acids, decreased IRF-1 expression, increased miR-126 activity, and diminished monocyte arrest.
Figures




References
-
- Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999;246:341–355. - PubMed
-
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and cardiovascular disease: A scientific statement from the american heart association. Circulation. 2011;123:2292–2333. - PubMed
-
- Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. - PubMed
-
- Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: Contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. - PubMed
-
- Chan JW, Motton D, Rutledge JC, Keim NL, Huser T. Raman spectroscopic analysis of biochemical changes in individual triglyceride-rich lipoproteins in the pre- and postprandial state. Analytical chemistry. 2005;77:5870–5876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

