CD47 deficiency confers cell and tissue radioprotection by activation of autophagy
- PMID: 22874555
- PMCID: PMC3494592
- DOI: 10.4161/auto.21562
CD47 deficiency confers cell and tissue radioprotection by activation of autophagy
Abstract
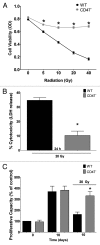
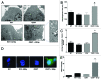
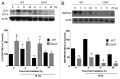
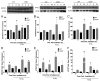
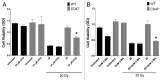
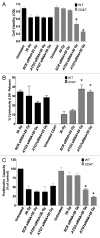
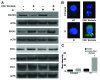
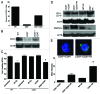
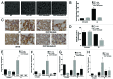
Accidental or therapeutic exposure to ionizing radiation has severe physiological consequences and can result in cell death. We previously demonstrated that deficiency or blockade of the ubiquitously expressed receptor CD47 results in remarkable cell and tissue protection against ischemic and radiation stress. Antagonists of CD47 or its ligand THBS1/thrombospondin 1 enhance cell survival and preserve their proliferative capacity. However the signaling pathways that mediate this cell-autonomous radioprotection are unclear. We now report a marked increase in autophagy in irradiated T-cells and endothelial cells lacking CD47. Irradiated T cells lacking CD47 exhibit significant increases in formation of autophagosomes comprising double-membrane vesicles visualized by electron microscopy and numbers of MAP1LC3A/B(+) puncta. Moreover, we observed significant increases in BECN1, ATG5, ATG7 and a reduction in SQSTM1/p62 expression relative to irradiated wild-type T cells. We observed similar increases in autophagy gene expression in mice resulting from blockade of CD47 in combination with total body radiation. Pharmacological or siRNA-mediated inhibition of autophagy selectively sensitized CD47-deficient cells to radiation, indicating that enhanced autophagy is necessary for the prosurvival response to CD47 blockade. Moreover, re-expression of CD47 in CD47-deficient T cells sensitized these cells to death by ionizing radiation and reversed the increase in autophagic flux associated with survival. This study indicates that CD47 deficiency confers cell survival through the activation of autophagic flux and identifies CD47 blockade as a pharmacological route to modulate autophagy for protecting tissue from radiation injury.
Keywords: ATG5; ATG7; BECN1; CD47; MAP1A/1BLC3; autophagosome; ionizing radiation; p62.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
