A reproducible mouse model of chronic allograft nephropathy with vasculopathy
- PMID: 22874842
- PMCID: PMC3495090
- DOI: 10.1038/ki.2012.277
A reproducible mouse model of chronic allograft nephropathy with vasculopathy
Abstract
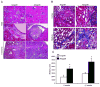
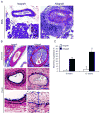
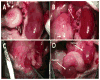
Although short-term outcomes in kidney transplantation have improved dramatically, long-term survival remains a major challenge. A key component of long-term, chronic allograft injury in solid organ transplants is arteriosclerosis characterized by vascular neointimal hyperplasia and inflammation. Establishing a model of this disorder would provide a unique tool not only to identify mechanisms of disease but also to test potential therapeutics for late graft injury. To this end, we utilized a mouse orthotopic renal transplant model in which C57BL/6J (H-2b) recipients were given either a kidney allograft from a completely mismatched Balb/cJ mouse (H-2d) or an isograft from a littermate. A unilateral nephrectomy was performed at the time of transplant followed by a contralateral nephrectomy on post-transplant day 7. Recipients were treated with daily cyclosporine subcutaneously for 14 days and then studied 8 and 12 weeks post transplantation. Renal function was significantly worse in allograft compared with isograft recipients. Moreover, the allografts had significantly more advanced tubulointerstitial fibrosis and profound vascular disease characterized by perivascular leukocytic infiltration and neointimal hyperplasia affecting the intrarenal blood vessels. Thus, we describe a feasible and reproducible murine model of intrarenal transplant arteriosclerosis that is useful to study allograft vasculopathy.
Figures

References
-
- Nankivell BJ, Kuypers DR. Diagnosis and prevention of chronic kidney allograft loss. Lancet. 2011;378:1428–1437. - PubMed
-
- Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–519. - PubMed
-
- Djamali A, Premasathian N, Pirsch JD. Outcomes in kidney transplantation. Semin Nephrol. 2003;23:306–316. - PubMed
-
- Rahmani M, Cruz RP, Granville DJ, et al. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

