Altered generation of induced regulatory T cells in the FVB.mdr1a-/- mouse model of colitis
- PMID: 22874899
- PMCID: PMC3676969
- DOI: 10.1038/mi.2012.73
Altered generation of induced regulatory T cells in the FVB.mdr1a-/- mouse model of colitis
Abstract
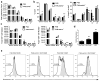
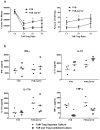
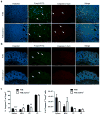
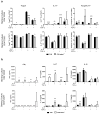
The FVB.mdr1a(-/-) mouse, lacking the small molecule pump P-glycoprotein (P-gp), is a commonly used model for the study of spontaneous T cell-mediated colitis. In addition, MDR1 polymorphisms and P-gp deficiency in humans have been linked to the development of ulcerative colitis. We now demonstrate that mice with P-gp deficiency have decreased levels of Foxp3(+) regulatory T cells (Tregs) in the intestinal lamina propria. This decrease is not due to either increased Treg apoptosis, altered Treg trafficking, or enhanced Treg plasticity to become Foxp3(+)IL-17(+) cells. Instead, P-gp deficiency appears to restrict the development of induced Treg cells (iTregs), as fewer Foxp3(+) iTregs developed from naive FVB.mdr1a(-/-) T cells both upon transforming growth factor-β (TGF-β) treatment in vitro and after adoptive transfer into FVB.rag2(-/-) recipients. Rather, in vitro TGF-β treatment results in a IL-17(+)CD4(+) T cell. This failure of iTregs to develop explains the decrease in Foxp3(+) Tregs in the FVB.mdr1a(-/-) intestine, representing a need to investigate this novel disease mechanism in human inflammatory bowel disease patients with MDR1 polymorphisms.
Conflict of interest statement
The authors declare no conflict of interest.
The authors declare no conflict of interest.
Figures


Similar articles
-
Critical role for P-glycoprotein expression in hematopoietic cells in the FVB.Mdr1a(-/-) model of colitis.J Pediatr Gastroenterol Nutr. 2011 Dec;53(6):666-73. doi: 10.1097/MPG.0b013e31822860f1. J Pediatr Gastroenterol Nutr. 2011. PMID: 21681110 Free PMC article.
-
Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4+CD25+Foxp3+ and CD4+CD25-Foxp3+ T cells.J Transl Med. 2019 Jul 9;17(1):219. doi: 10.1186/s12967-019-1967-3. J Transl Med. 2019. PMID: 31288845 Free PMC article.
-
Loss of TLR2 worsens spontaneous colitis in MDR1A deficiency through commensally induced pyroptosis.J Immunol. 2013 Jun 1;190(11):5676-88. doi: 10.4049/jimmunol.1201592. Epub 2013 May 1. J Immunol. 2013. PMID: 23636052 Free PMC article.
-
A dynamic dual role of IL-2 signaling in the two-step differentiation process of adaptive regulatory T cells.J Immunol. 2013 Apr 1;190(7):3153-62. doi: 10.4049/jimmunol.1200751. Epub 2013 Feb 20. J Immunol. 2013. PMID: 23427250 Free PMC article.
-
Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other.Trends Immunol. 2008 Sep;29(9):429-35. doi: 10.1016/j.it.2008.06.005. Epub 2008 Aug 3. Trends Immunol. 2008. PMID: 18676178 Review.
Cited by
-
Animal models of ulcerative colitis and their application in drug research.Drug Des Devel Ther. 2013 Nov 12;7:1341-57. doi: 10.2147/DDDT.S40107. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 24250223 Free PMC article. Review.
-
A Review of the Scaffold Protein Menin and its Role in Hepatobiliary Pathology.Gene Expr. 2017 Jul 7;17(3):251-263. doi: 10.3727/105221617X695744. Epub 2017 Apr 28. Gene Expr. 2017. PMID: 28485270 Free PMC article. Review.
-
P-glycoprotein and drug resistance in systemic autoimmune diseases.Int J Mol Sci. 2014 Mar 20;15(3):4965-76. doi: 10.3390/ijms15034965. Int J Mol Sci. 2014. PMID: 24658440 Free PMC article. Review.
-
P-glycoprotein: new insights into structure, physiological function, regulation and alterations in disease.Heliyon. 2022 Jun 22;8(6):e09777. doi: 10.1016/j.heliyon.2022.e09777. eCollection 2022 Jun. Heliyon. 2022. PMID: 35789865 Free PMC article. Review.
-
P-glycoprotein expression skews mitochondrial dye measurements in T cells.Front Immunol. 2025 Jun 18;16:1560104. doi: 10.3389/fimmu.2025.1560104. eCollection 2025. Front Immunol. 2025. PMID: 40607427 Free PMC article.
References
-
- Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. - PubMed
-
- Schinkel AH, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. - PubMed
-
- Johnstone RW, Cretney E, Smyth MJ. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood. 1999;93:1075–1085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

