Pten deletion causes mTorc1-dependent ectopic neuroblast differentiation without causing uniform migration defects
- PMID: 22874917
- PMCID: PMC3424045
- DOI: 10.1242/dev.083154
Pten deletion causes mTorc1-dependent ectopic neuroblast differentiation without causing uniform migration defects
Abstract
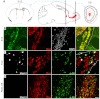
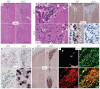
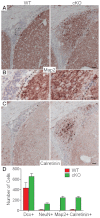
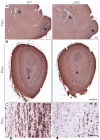
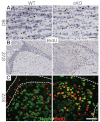
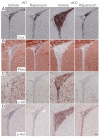
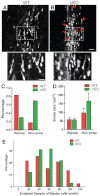
Neuronal precursors, generated throughout life in the subventricular zone, migrate through the rostral migratory stream to the olfactory bulb where they differentiate into interneurons. We found that the PI3K-Akt-mTorc1 pathway is selectively inactivated in migrating neuroblasts in the subventricular zone and rostral migratory stream, and activated when these cells reach the olfactory bulb. Postnatal deletion of Pten caused aberrant activation of the PI3K-Akt-mTorc1 pathway and an enlarged subventricular zone and rostral migratory stream. This expansion was caused by premature termination of migration and differentiation of neuroblasts and was rescued by inhibition of mTorc1. This phenotype is reminiscent of lamination defects caused by Pten deletion in developing brain that were previously described as defective migration. However, live imaging in acute slices showed that Pten deletion did not cause a uniform defect in the mechanics of directional neuroblast migration. Instead, a subpopulation of Pten-null neuroblasts showed minimal movement and altered morphology associated with differentiation, whereas the remainder showed unimpeded directional migration towards the olfactory bulb. Therefore, migration defects of Pten-null neurons might be secondary to ectopic differentiation.
Figures
Similar articles
-
Dynamic Changes in Ultrastructure of the Primary Cilium in Migrating Neuroblasts in the Postnatal Brain.J Neurosci. 2019 Dec 11;39(50):9967-9988. doi: 10.1523/JNEUROSCI.1503-19.2019. Epub 2019 Nov 4. J Neurosci. 2019. PMID: 31685650 Free PMC article.
-
Rheb activation in subventricular zone progenitors leads to heterotopia, ectopic neuronal differentiation, and rapamycin-sensitive olfactory micronodules and dendrite hypertrophy of newborn neurons.J Neurosci. 2013 Feb 6;33(6):2419-31. doi: 10.1523/JNEUROSCI.1840-12.2013. J Neurosci. 2013. PMID: 23392671 Free PMC article.
-
RhoE deficiency alters postnatal subventricular zone development and the number of calbindin-expressing neurons in the olfactory bulb of mouse.Brain Struct Funct. 2015 Nov;220(6):3113-30. doi: 10.1007/s00429-014-0846-1. Epub 2014 Jul 10. Brain Struct Funct. 2015. PMID: 25009316
-
Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review.
-
Extracellular signals controlling neuroblast migration in the postnatal brain.Adv Exp Med Biol. 2014;800:149-80. doi: 10.1007/978-94-007-7687-6_9. Adv Exp Med Biol. 2014. PMID: 24243105 Review.
Cited by
-
Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain.Nat Cell Biol. 2013 Jun;15(6):614-24. doi: 10.1038/ncb2735. Epub 2013 May 5. Nat Cell Biol. 2013. PMID: 23644469 Free PMC article.
-
The role of mTOR signalling in neurogenesis, insights from tuberous sclerosis complex.Semin Cell Dev Biol. 2016 Apr;52:12-20. doi: 10.1016/j.semcdb.2016.01.040. Epub 2016 Feb 2. Semin Cell Dev Biol. 2016. PMID: 26849906 Free PMC article. Review.
-
Phosphatase and Tensin Homologue: Novel Regulation by Developmental Signaling.J Signal Transduct. 2015;2015:282567. doi: 10.1155/2015/282567. Epub 2015 Aug 3. J Signal Transduct. 2015. PMID: 26339505 Free PMC article. Review.
-
Somatic mosaicism and interneuron involvement in mTORopathies.Trends Neurosci. 2025 May;48(5):362-376. doi: 10.1016/j.tins.2025.02.009. Epub 2025 Mar 22. Trends Neurosci. 2025. PMID: 40121168 Review.
-
Neurobiology of autism gene products: towards pathogenesis and drug targets.Psychopharmacology (Berl). 2014 Mar;231(6):1037-62. doi: 10.1007/s00213-013-3403-3. Epub 2014 Jan 14. Psychopharmacology (Berl). 2014. PMID: 24419271 Review.
References
-
- Alvarez-Buylla A., Kohwi M., Nguyen T. M., Merkle F. T. (2008). The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb. Symp. Quant. Biol. 73, 357–365 - PubMed
-
- Anton E. S., Ghashghaei H. T., Weber J. L., McCann C., Fischer T. M., Cheung I. D., Gassmann M., Messing A., Klein R., Schwab M. H., et al. (2004). Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat. Neurosci. 7, 1319–1328 - PubMed
-
- Backman S. A., Stambolic V., Suzuki A., Haight J., Elia A., Pretorius J., Tsao M. S., Shannon P., Bolon B., Ivy G. O., et al. (2001). Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29, 396–403 - PubMed
-
- Bateman J. M., McNeill H. (2004). Temporal control of differentiation by the insulin receptor/tor pathway in Drosophila. Cell 119, 87–96 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

