Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B
- PMID: 22875003
- PMCID: PMC3434657
- DOI: 10.1038/cddis.2012.109
Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B
Abstract
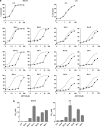
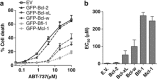
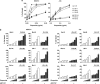
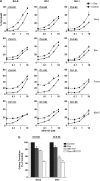
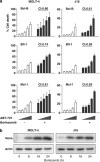
The novel anticancer drug ABT-737 is a Bcl-2 Homology 3 (BH3)-mimetic that induces apoptosis by inhibiting pro-survival Bcl-2 proteins. ABT-737 binds with equal affinity to Bcl-2, Bcl-xL and Bcl-w in vitro and is expected to overrule apoptosis resistance mediated by these Bcl-2 proteins in equal measure. We have profiled ABT-737 specificity for all six pro-survival Bcl-2 proteins, in p53 wild-type or p53-mutant human T-leukemic cells. Bcl-B was untargeted, like Bfl-1 and Mcl-1, in accord with their low affinity for ABT-737 in vitro. However, Bcl-2 proved a better ABT-737 target than Bcl-xL and Bcl-w. This was reflected in differential apoptosis-sensitivity to ABT-737 alone, or combined with etoposide. ABT-737 was not equally effective in displacing BH3-only proteins or Bax from Bcl-2, as compared with Bcl-xL or Bcl-w, offering an explanation for the differential ABT-737 sensitivity of tumor cells overexpressing these proteins. Inducible expression demonstrated that BH3-only proteins Noxa, but not Bim, Puma or truncated Bid could overrule ABT-737 resistance conferred by Bcl-B, Bfl-1 or Mcl-1. These data identify Bcl-B, Bfl-1 and Mcl-1, but also Bcl-xL and Bcl-w as potential mediators of ABT-737 resistance and indicate that target proteins can be differentially sensitive to BH3-mimetics, depending on the pro-apoptotic Bcl-2 proteins they are complexed with.
Figures

Similar articles
-
Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells.Blood. 2012 Jun 14;119(24):5807-16. doi: 10.1182/blood-2011-12-400929. Epub 2012 Apr 26. Blood. 2012. PMID: 22538851 Free PMC article.
-
Mcl-1 Phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells.Cancer Res. 2012 Jun 15;72(12):3069-79. doi: 10.1158/0008-5472.CAN-11-4106. Epub 2012 Apr 23. Cancer Res. 2012. PMID: 22525702 Free PMC article.
-
Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice.Cell Death Differ. 2019 Jul;26(7):1316-1331. doi: 10.1038/s41418-018-0209-1. Epub 2018 Nov 23. Cell Death Differ. 2019. PMID: 30470795 Free PMC article.
-
BH3 mimetics to improve cancer therapy; mechanisms and examples.Drug Resist Updat. 2007 Dec;10(6):207-17. doi: 10.1016/j.drup.2007.08.002. Epub 2007 Oct 24. Drug Resist Updat. 2007. PMID: 17921043 Free PMC article. Review.
-
Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy?Cell Death Differ. 2008 Jun;15(6):977-87. doi: 10.1038/cdd.2008.37. Epub 2008 Mar 28. Cell Death Differ. 2008. PMID: 18369371 Free PMC article. Review.
Cited by
-
Vinblastine rapidly induces NOXA and acutely sensitizes primary chronic lymphocytic leukemia cells to ABT-737.Mol Cancer Ther. 2013 Aug;12(8):1504-14. doi: 10.1158/1535-7163.MCT-12-1197. Epub 2013 May 30. Mol Cancer Ther. 2013. PMID: 23723123 Free PMC article.
-
Polyubiquitination and proteasomal turnover controls the anti-apoptotic activity of Bcl-B.Oncogene. 2013 Nov 28;32(48):5439-48. doi: 10.1038/onc.2013.99. Epub 2013 Apr 8. Oncogene. 2013. PMID: 23563182 Free PMC article.
-
Pharmacological regulators of autophagy and their link with modulators of lupus disease.Br J Pharmacol. 2014 Oct;171(19):4337-59. doi: 10.1111/bph.12792. Epub 2014 Sep 5. Br J Pharmacol. 2014. PMID: 24902607 Free PMC article. Review.
-
ABT-737 ameliorates docetaxel resistance in triple negative breast cancer cell line.Ann Surg Treat Res. 2018 Nov;95(5):240-248. doi: 10.4174/astr.2018.95.5.240. Epub 2018 Oct 25. Ann Surg Treat Res. 2018. PMID: 30402442 Free PMC article.
-
BCL-w: apoptotic and non-apoptotic role in health and disease.Cell Death Dis. 2020 Apr 21;11(4):260. doi: 10.1038/s41419-020-2417-0. Cell Death Dis. 2020. PMID: 32317622 Free PMC article. Review.
References
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. - PubMed
-
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. - PubMed
-
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

