Functional genomics identifies drivers of medulloblastoma dissemination
- PMID: 22875024
- PMCID: PMC3463769
- DOI: 10.1158/0008-5472.CAN-12-1629
Functional genomics identifies drivers of medulloblastoma dissemination
Abstract
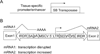
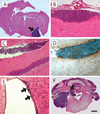
Medulloblastomas are malignant brain tumors that arise in the cerebellum in children and disseminate via the cerebrospinal fluid to the leptomeningeal spaces of the brain and spinal cord. Challenged by the poor prognosis for patients with metastatic dissemination, pediatric oncologists have developed aggressive treatment protocols, combining surgery, craniospinal radiation, and high-dose chemotherapy, that often cause disabling neurotoxic effects in long-term survivors. Insights into the genetic control of medulloblastoma dissemination have come from transposon insertion mutagenesis studies. Mobilizing the Sleeping Beauty transposon in cerebellar neural progenitor cells caused widespread dissemination of typically nonmetastatic medulloblastomas in Patched(+/-) mice, in which Shh signaling is hyperactive. Candidate metastasis genes were identified by sequencing the insertion sites and then mapping these sequences back to the mouse genome. To determine whether genes located at transposon insertion sites directly caused medulloblastomas to disseminate, we overexpressed candidate genes in Nestin(+) neural progenitors in the cerebella of mice by retroviral transfer in combination with Shh. We show here that ectopic expression of Eras, Lhx1, Ccrk, and Akt shifted the in vivo growth characteristics of Shh-induced medulloblastomas from a localized pattern to a disseminated pattern in which tumor cells seeded the leptomeningeal spaces of the brain and spinal cord.
©2012 AACR.
Conflict of interest statement
Conflict of interest statement: The authors disclose no potential conflicts of interest.
Figures



Similar articles
-
Genetic drivers of metastatic dissemination in sonic hedgehog medulloblastoma.Acta Neuropathol Commun. 2014 Jul 25;2:85. doi: 10.1186/s40478-014-0085-y. Acta Neuropathol Commun. 2014. PMID: 25059231 Free PMC article.
-
c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice.Neoplasia. 2003 May-Jun;5(3):198-204. doi: 10.1016/S1476-5586(03)80052-0. Neoplasia. 2003. PMID: 12869303 Free PMC article.
-
Hepatocyte growth factor and sonic Hedgehog expression in cerebellar neural progenitor cells costimulate medulloblastoma initiation and growth.Cancer Res. 2008 Oct 1;68(19):7838-45. doi: 10.1158/0008-5472.CAN-08-1899. Cancer Res. 2008. PMID: 18829539 Free PMC article.
-
Medulloblastoma: Molecular Classification-Based Personal Therapeutics.Neurotherapeutics. 2017 Apr;14(2):265-273. doi: 10.1007/s13311-017-0526-y. Neurotherapeutics. 2017. PMID: 28386677 Free PMC article. Review.
-
Medulloblastomics: the end of the beginning.Nat Rev Cancer. 2012 Dec;12(12):818-34. doi: 10.1038/nrc3410. Nat Rev Cancer. 2012. PMID: 23175120 Free PMC article. Review.
Cited by
-
Sleeping Beauty Mouse Models of Cancer: Microenvironmental Influences on Cancer Genetics.Front Oncol. 2019 Jul 9;9:611. doi: 10.3389/fonc.2019.00611. eCollection 2019. Front Oncol. 2019. PMID: 31338332 Free PMC article. Review.
-
Computer-assisted quantification of motile and invasive capabilities of cancer cells.Sci Rep. 2015 Oct 21;5:15338. doi: 10.1038/srep15338. Sci Rep. 2015. PMID: 26486848 Free PMC article.
-
PTEN restrains SHH medulloblastma growth through cell autonomous and nonautonomous mechanisms.bioRxiv [Preprint]. 2025 Aug 2:2025.07.31.667996. doi: 10.1101/2025.07.31.667996. bioRxiv. 2025. PMID: 40766638 Free PMC article. Preprint.
-
Sleeping Beauty transposon system for genetic etiological research and gene therapy of cancers.Cancer Biol Ther. 2015;16(1):8-16. doi: 10.4161/15384047.2014.986944. Cancer Biol Ther. 2015. PMID: 25455252 Free PMC article. Review.
-
DiSCoVERing Innovative Therapies for Rare Tumors: Combining Genetically Accurate Disease Models with In Silico Analysis to Identify Novel Therapeutic Targets.Clin Cancer Res. 2016 Aug 1;22(15):3903-14. doi: 10.1158/1078-0432.CCR-15-3011. Epub 2016 Mar 24. Clin Cancer Res. 2016. PMID: 27012813 Free PMC article.
References
-
- Pollack IF. Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances, and challenges. J Neurosurg Pediatr. 2011;8:135–148. - PubMed
-
- Radcliffe J, Packer RJ, Atkins TE, Bunin GR, Schut L, Goldwein JW, et al. Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole-brain radiotherapy. Ann Neurol. 1992;32:551–554. - PubMed
-
- Silber JH, Littman PS, Meadows AT. Stature loss following skeletal irradiation for childhood cancer. J Clin Oncol. 1990;8:304–312. - PubMed
-
- Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

