Microbial D-xylonate production
- PMID: 22875400
- PMCID: PMC3433669
- DOI: 10.1007/s00253-012-4288-5
Microbial D-xylonate production
Abstract
D-Xylonic acid is a versatile platform chemical with reported applications as complexing agent or chelator, in dispersal of concrete, and as a precursor for compounds such as co-polyamides, polyesters, hydrogels and 1,2,4-butanetriol. With increasing glucose prices, D-xylonic acid may provide a cheap, non-food derived alternative for gluconic acid, which is widely used (about 80 kton/year) in pharmaceuticals, food products, solvents, adhesives, dyes, paints and polishes. Large-scale production has not been developed, reflecting the current limited market for D-xylonate. D-Xylonic acid occurs naturally, being formed in the first step of oxidative metabolism of D-xylose by some archaea and bacteria via the action of D-xylose or D-glucose dehydrogenases. High extracellular concentrations of D-xylonate have been reported for various bacteria, in particular Gluconobacter oxydans and Pseudomonas putida. High yields of D-xylonate from D-xylose make G. oxydans an attractive choice for biotechnical production. G. oxydans is able to produce D-xylonate directly from plant biomass hydrolysates, but rates and yields are reduced because of sensitivity to hydrolysate inhibitors. Recently, D-xylonate has been produced by the genetically modified bacterium Escherichia coli and yeast Saccharomyces cerevisiae and Kluyveromyces lactis. Expression of NAD(+)-dependent D-xylose dehydrogenase of Caulobacter crescentus in either E. coli or in a robust, hydrolysate-tolerant, industrial Saccharomyces cerevisiae strain has resulted in D-xylonate titres, which are comparable to those seen with G. oxydans, at a volumetric rate approximately 30% of that observed with G. oxydans. With further development, genetically modified microbes may soon provide an alternative for production of D-xylonate at industrial scale.
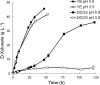
Figures




Similar articles
-
Low pH D-xylonate production with Pichia kudriavzevii.Bioresour Technol. 2013 Apr;133:555-62. doi: 10.1016/j.biortech.2013.01.157. Epub 2013 Feb 7. Bioresour Technol. 2013. PMID: 23455228
-
A novel aldose-aldose oxidoreductase for co-production of D-xylonate and xylitol from D-xylose with Saccharomyces cerevisiae.Appl Microbiol Biotechnol. 2015 Nov;99(22):9439-47. doi: 10.1007/s00253-015-6878-5. Epub 2015 Aug 12. Appl Microbiol Biotechnol. 2015. PMID: 26264136 Free PMC article.
-
Metabolic engineering of Escherichia coli for the production of xylonate.PLoS One. 2013 Jul 5;8(7):e67305. doi: 10.1371/journal.pone.0067305. Print 2013. PLoS One. 2013. PMID: 23861757 Free PMC article.
-
Everyone loves an underdog: metabolic engineering of the xylose oxidative pathway in recombinant microorganisms.Appl Microbiol Biotechnol. 2018 Sep;102(18):7703-7716. doi: 10.1007/s00253-018-9186-z. Epub 2018 Jul 12. Appl Microbiol Biotechnol. 2018. PMID: 30003296 Review.
-
[Engineering of the xylose metabolic pathway for microbial production of bio-based chemicals].Sheng Wu Gong Cheng Xue Bao. 2013 Aug;29(8):1161-72. Sheng Wu Gong Cheng Xue Bao. 2013. PMID: 24364352 Review. Chinese.
Cited by
-
Recent progress in the microbial production of xylonic acid.World J Microbiol Biotechnol. 2022 Jun 7;38(7):127. doi: 10.1007/s11274-022-03313-5. World J Microbiol Biotechnol. 2022. PMID: 35668329 Review.
-
Transcriptome of Saccharomyces cerevisiae during production of D-xylonate.BMC Genomics. 2014 Sep 5;15(1):763. doi: 10.1186/1471-2164-15-763. BMC Genomics. 2014. PMID: 25192596 Free PMC article.
-
Global Transcriptome Profile of the Oleaginous Yeast Saitozyma podzolica DSM 27192 Cultivated in Glucose and Xylose.J Fungi (Basel). 2021 Sep 15;7(9):758. doi: 10.3390/jof7090758. J Fungi (Basel). 2021. PMID: 34575796 Free PMC article.
-
Genome-scale metabolic network reconstructions of diverse Escherichia strains reveal strain-specific adaptations.Philos Trans R Soc Lond B Biol Sci. 2022 Oct 10;377(1861):20210236. doi: 10.1098/rstb.2021.0236. Epub 2022 Aug 22. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 35989599 Free PMC article.
-
Biotransformation of d-xylose to d-xylonate coupled to medium-chain-length polyhydroxyalkanoate production in cellobiose-grown Pseudomonas putida EM42.Microb Biotechnol. 2020 Jul;13(4):1273-1283. doi: 10.1111/1751-7915.13574. Epub 2020 May 3. Microb Biotechnol. 2020. PMID: 32363744 Free PMC article.
References
-
- Attwood MM, Vandijken JP, Pronk JT. Glucose-metabolism and gluconic acid production by Acetobacter diazotrophicus. J Ferment Bioeng. 1991;72:101–105. doi: 10.1016/0922-338X(91)90317-A. - DOI
-
- Brouns SJ, Walther J, Snijders AP, van de Werken HJ, Willemen HL, Worm P, de Vos MG, Andersson A, Lundgren M, Mazon HF, van den Heuvel RH, Nilsson P, Salmon L, de Vos WM, Wright PC, Bernander R, van der Oost J. Identification of the missing links in prokaryotic pentose oxidation pathways: evidence for enzyme recruitment. J Biol Chem. 2006;281:27378–27388. doi: 10.1074/jbc.M605549200. - DOI - PubMed
-
- Buchert J (1990) Biotechnical oxidation of D-xylose and hemicellulose hydrolyzates by Gluconobacter oxydans. Dissertation, Helsinki University of Technology
-
- Buchert J, Viikari L. The role of xylonolactone in xylonic acid production by Pseudomonas fragi. Appl Microbiol Biotechnol. 1988;27:333–336. doi: 10.1007/BF00251763. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

