Has the time for first-line treatment with second generation tyrosine kinase inhibitors in patients with chronic myelogenous leukemia already come? Systematic review and meta-analysis
- PMID: 22875617
- PMCID: PMC3533665
- DOI: 10.3324/haematol.2012.063172
Has the time for first-line treatment with second generation tyrosine kinase inhibitors in patients with chronic myelogenous leukemia already come? Systematic review and meta-analysis
Abstract
Second generation tyrosine kinase inhibitors have recently been introduced as first-line treatment for chronic phase chronic myelogenous leukemia. We aimed to evaluate the efficacy and safety of 2(nd) generation tyrosine kinase inhibitors versus imatinib as first-line treatment for these patients. We carried out a systematic review and meta-analysis of randomized controlled trials comparing 2(nd) generation tyrosine kinase inhibitors to imatinib as first-line treatment in chronic phase chronic myelogenous leukemia patients. Outcomes assessed were: complete cytogenetic response and major molecular response at 12, 18 and 24 months, all-cause mortality and progression to accelerated phase/blastic crisis at 12, 18 and 24 months, and chronic myelogenous leukemia related mortality and toxicity at last follow up. Relative risks were estimated and pooled using a fixed effect model. Our search yielded four trials including 2,120 patients. At 12 months, treatment with 2(nd) generation tyrosine kinase inhibitors significantly improved both complete cytogenetic response and major molecular response (relative risk 1.16, 95% CI: 1.09-1.23, and 1.68, 95% CI: 1.48-1.91, respectively). While major molecular response was improved at all time points, complete cytogenetic response improved at 18 months but not at 24 months. Importantly, rate of progression to accelerated phase/blastic crisis was significantly lower with the newer tyrosine kinase inhibitors throughout all time points. Second generation tyrosine kinase inhibitors improved chronic myelogenous leukemia related mortality without a statistically significant difference in all-cause mortality at 12, 18 and 24 months. We conclude that 2(nd) generation tyrosine kinase inhibitors can be added safely to the first-line treatment armamentarium of chronic phase chronic myelogenous leukemia patients. Although an advantage is suggested by surrogate parameters, longer follow up is necessary to see if this translates into superior overall survival.
Figures
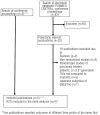




References
-
- Sawyers CL. hronic myeloid leukemia. N Engl J Med. 1999;340(17):1330-40 - PubMed
-
- O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994-1004 - PubMed
-
- Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349(15):1423-32 - PubMed
-
- Deininger M, O'Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. ASH Annual Meeting Abstracts. 2009;114:462
-
- Saussele S, Pfirrmann M. Clinical trials in chronic myeloid leukemia. Curr Hematol Malig Rep. 2012;7(2):109-15 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

