Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy
- PMID: 22875622
- PMCID: PMC3561425
- DOI: 10.3324/haematol.2012.066480
Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy
Abstract
Imatinib has so far been the first-choice treatment in chronic myeloid leukemia with excellent results. However, only a proportion of patients achieve major molecular response - hence the need to find biological predictors of outcome to select the optimal therapeutic strategy now that more potent inhibitors are available. We investigated a panel of 20 polymorphisms in seven genes, potentially associated with the pharmacogenetics of imatinib, in a subset of 189 patients with newly diagnosed chronic myeloid leukemia enrolled in the TOPS trial. The analysis included polymorphisms in the transporters hOCT1, MDR1, ABCG2, OCTN1, and OATP1A2, and in the metabolizing genes CYP3A4 and CYP3A5. In the overall population, the OCTN1 C allele (rs1050152), a simple combination of polymorphisms in the hOCT1 gene and another combination in the genes involved in imatinib uptake were significantly associated with major molecular response. The combination of polymorphisms in imatinib uptake was also significantly associated with complete molecular response. Analyses restricted to Caucasians highlighted the significant association of MDR1 CC (rs60023214) genotype with complete molecular response. We demonstrate the usefulness of a pharmacogenetic approach for stratifying patients with chronic myeloid leukemia according to their likelihood of achieving a major or complete molecular response to imatinib. This represents an attractive opportunity for therapy optimization, worth testing in clinical trials.
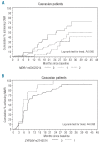
Figures


References
-
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-17 - PubMed
-
- Hughes TP, Branford S. Monitoring disease response to tyrosine kinase inhibitor therapy in CML. Hematology Am Soc Hematol Educ Program. 2009;477-87 - PubMed
-
- Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58-60 - PubMed
-
- Haiat S, Decleves X, Mittaine B, Legrand O, Francart S, Rio B, et al. Determination of imatinib plasma levels by high performance liquid chromatography (HPLC): evaluation of pharmacokinetic variability and haematological consequences. Blood. 2006;108 Abstract 1375.
-
- Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44(9):879-94 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

