Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4
- PMID: 22875785
- PMCID: PMC3469600
- DOI: 10.1152/ajpcell.00457.2011
Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4
Abstract
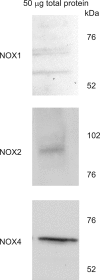
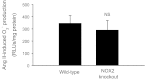
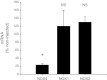
Angiotensin II (ANG II) stimulates production of superoxide (O(2)(-)) by NADPH oxidase (NOX) in medullary thick ascending limbs (TALs). There are three isoforms of the catalytic subunit (NOX1, 2, and 4) known to be expressed in the kidney. We hypothesized that NOX2 mediates ANG II-induced O(2)(-) production by TALs. To test this, we measured NOX1, 2, and 4 mRNA and protein by RT-PCR and Western blot in TAL suspensions from rats and found three catalytic subunits expressed in the TAL. We measured O(2)(-) production using a lucigenin-based assay. To assess the contribution of NOX2, we measured ANG II-induced O(2)(-) production in wild-type and NOX2 knockout mice (KO). ANG II increased O(2)(-) production by 346 relative light units (RLU)/mg protein in the wild-type mice (n = 9; P < 0.0007 vs. control). In the knockout mice, ANG II increased O(2)(-) production by 290 RLU/mg protein (n = 9; P < 0.007 vs. control). This suggests that NOX2 does not contribute to ANG II-induced O(2)(-) production (P < 0.6 WT vs. KO). To test whether NOX4 mediates the effect of ANG II, we selectively decreased NOX4 expression in rats using an adenovirus that expresses NOX4 short hairpin (sh)RNA. Six to seven days after in vivo transduction of the kidney outer medulla, NOX4 mRNA was reduced by 77%, while NOX1 and NOX2 mRNA was unaffected. In control TALs, ANG II stimulated O(2)(-) production by 96%. In TALs transduced with NOX4 shRNA, ANG II-stimulated O(2)(-) production was not significantly different from the baseline. We concluded that NOX4 is the main catalytic isoform of NADPH oxidase that contributes to ANG II-stimulated O(2)(-) production by TALs.
Figures





Similar articles
-
Angiotensin II stimulates superoxide production by nitric oxide synthase in thick ascending limbs.Physiol Rep. 2016 Feb;4(4):e12697. doi: 10.14814/phy2.12697. Physiol Rep. 2016. PMID: 26884476 Free PMC article.
-
NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs.Am J Physiol Renal Physiol. 2012 Oct 15;303(8):F1151-6. doi: 10.1152/ajprenal.00181.2012. Epub 2012 Aug 15. Am J Physiol Renal Physiol. 2012. PMID: 22896039 Free PMC article.
-
Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(α)-dependent NADPH oxidase activation.J Biol Chem. 2010 Jul 9;285(28):21323-8. doi: 10.1074/jbc.M110.109157. Epub 2010 May 6. J Biol Chem. 2010. PMID: 20448043 Free PMC article.
-
Angiotensin II, NADPH oxidase, and redox signaling in the vasculature.Antioxid Redox Signal. 2013 Oct 1;19(10):1110-20. doi: 10.1089/ars.2012.4641. Epub 2012 Jun 11. Antioxid Redox Signal. 2013. PMID: 22530599 Free PMC article. Review.
-
Unchanged NADPH Oxidase Activity in Nox1-Nox2-Nox4 Triple Knockout Mice: What Do NADPH-Stimulated Chemiluminescence Assays Really Detect?Antioxid Redox Signal. 2016 Mar 1;24(7):392-9. doi: 10.1089/ars.2015.6314. Epub 2015 Jun 30. Antioxid Redox Signal. 2016. PMID: 25906178 Review.
Cited by
-
Mechanisms of decreased tubular flow-induced nitric oxide in Dahl salt-sensitive rat thick ascending limbs.Am J Physiol Renal Physiol. 2021 Sep 1;321(3):F369-F377. doi: 10.1152/ajprenal.00124.2021. Epub 2021 Jul 26. Am J Physiol Renal Physiol. 2021. PMID: 34308669 Free PMC article.
-
Angiotensin II stimulates basolateral 50-pS K channels in the thick ascending limb.Am J Physiol Renal Physiol. 2014 Mar 1;306(5):F509-16. doi: 10.1152/ajprenal.00476.2013. Epub 2013 Dec 26. Am J Physiol Renal Physiol. 2014. PMID: 24370594 Free PMC article.
-
Angiotensin II stimulates superoxide production by nitric oxide synthase in thick ascending limbs.Physiol Rep. 2016 Feb;4(4):e12697. doi: 10.14814/phy2.12697. Physiol Rep. 2016. PMID: 26884476 Free PMC article.
-
Free radical scavenging reverses fructose-induced salt-sensitive hypertension.Integr Blood Press Control. 2017 Dec 19;11:1-9. doi: 10.2147/IBPC.S147674. eCollection 2018. Integr Blood Press Control. 2017. PMID: 29296095 Free PMC article.
-
Diabetes and Kidney Disease: Role of Oxidative Stress.Antioxid Redox Signal. 2016 Oct 20;25(12):657-684. doi: 10.1089/ars.2016.6664. Epub 2016 Apr 1. Antioxid Redox Signal. 2016. PMID: 26906673 Free PMC article. Review.
References
-
- Alvarez E, Rodino-Janeiro BK, Ucieda-Somoza R, Gonzalez-Juanatey JR. Pravastatin counteracts angiotensin II-induced upregulation and activation of NADPH oxidase at plasma membrane of human endothelial cells. J Cardiovasc Pharmacol 55: 203–212, 2010 - PubMed
-
- Bartsch C, Bekhite MM, Wolheim A, Richter M, Ruhe C, Wissuwa B, Marciniak A, Muller J, Heller R, Figulla HR, Sauer H, Wartenberg M. NADPH oxidase and eNOS control cardiomyogenesis in mouse embryonic stem cells on ascorbic acid treatment. Free Radic Biol Med 51: 432–443, 2011 - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

