Endoplasmic reticulum stress pathway required for immune homeostasis is neurally controlled by arrestin-1
- PMID: 22875856
- PMCID: PMC3460425
- DOI: 10.1074/jbc.M112.398362
Endoplasmic reticulum stress pathway required for immune homeostasis is neurally controlled by arrestin-1
Abstract
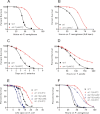
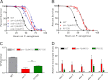
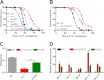
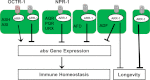
In response to pathogen infection, the host innate immune system activates microbial killing pathways and cellular stress pathways that need to be balanced because insufficient or excessive immune responses have deleterious consequences. Recent studies demonstrate that two G protein-coupled receptors (GPCRs) in the nervous system of Caenorhabditis elegans control immune homeostasis. To investigate further how GPCR signaling controls immune homeostasis at the organismal level, we studied arrestin-1 (ARR-1), which is the only GPCR adaptor protein in C. elegans. The results indicate that ARR-1 is required for GPCR signaling in ASH, ASI, AQR, PQR, and URX neurons, which control the unfolded protein response and a p38 mitogen-activated protein kinase signaling pathway required for innate immunity. ARR-1 activity also controlled immunity through ADF chemosensory and AFD thermosensory neurons that regulate longevity. Furthermore, we found that although ARR-1 played a key role in the control of immunity by AFD thermosensory neurons, it did not control longevity through these cells. However, ARR-1 partially controlled longevity through ADF neurons.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

