Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence
- PMID: 22875876
- PMCID: PMC6429561
- DOI: 10.1093/cid/cis616
Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence
Abstract
Background: Little is known about viral hepatitis testing and infection prevalence among persons in private healthcare organizations (HCOs) in the United States.
Methods: To determine the frequency of and characteristics associated with viral hepatitis testing and infection prevalence among adults with access to care, we conducted an observational cohort study among 1.25 million adults from 4 US HCOs and included persons with ≥1 clinical encounter during 2006-2008 and ≥12 months of continuous follow-up before 2009. We compared the number of infections identified with the number expected based on adjusted data from the National Health and Nutrition Examination Survey (NHANES).
Results: Of 866,886 persons without a previous hepatitis B virus (HBV) diagnosis, 18.8% were tested for HBV infection, of whom 1.4% tested positive; among 865,659 without a previous hepatitis C virus (HCV) diagnosis, 12.7% were tested, of whom 5.5% tested positive. Less than half of those with ≥2 abnormal alanine aminotransferase (ALT) levels were subsequently tested for HBV or HCV. When tested, Asians (adjusted odds ratio [aOR] 6.33 relative to whites) were most likely HBV infected, whereas those aged 50-59 years were most likely HCV infected (aOR 6.04, relative to age <30 years). Based on estimates from NHANES, nearly one-half of HCV and one-fifth of HBV infections in this population were not identified.
Conclusions: Even in this population with access to care and lengthy follow-up, only a fraction of expected viral hepatitis infections were identified. Abnormal ALT levels often but not consistently triggered testing. These findings have implications for the identification and care of 4-5 million US residents with HBV and HCV infection.
Conflict of interest statement
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
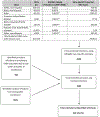

References
-
- IOM (Institute of Medicine). Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press, 2010. - PubMed
-
- CDC. Viral hepatitis: statistics and surveillance, 2009. Available at: http://www.cdc.gov/hepatitis/Statistics.htm. Accessed 19 May 2011.
-
- Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology 2007; 46:1034–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

