Type-specific identification of anogenital herpes simplex virus infections by use of a commercially available nucleic acid amplification test
- PMID: 22875892
- PMCID: PMC3486267
- DOI: 10.1128/JCM.01685-12
Type-specific identification of anogenital herpes simplex virus infections by use of a commercially available nucleic acid amplification test
Abstract
Herpes infections are among the most common sexually transmitted infections (STI), but diagnostic methods for genital herpes have not kept pace with the movement toward molecular testing. Here, we describe an FDA-approved molecular assay that identifies and types herpes simplex virus (HSV) infections for use in routine clinical settings. Paired samples from anogenital lesions were tested using the BD ProbeTec HSV Q(x) (HSVQ(x)) system, HSV culture and, a laboratory-developed PCR assay. Family planning, obstetrics/gynecology (OB/GYN), or sexually transmitted disease (STD) clinics in the United States served as recruitment sites. Sensitivity and specificity estimates, head-to-head comparisons, measures of agreement, and latent-class analyses were performed to provide robust estimates of performance. A total of 508 participants (174 men and 334 women) with anogenital lesions were included; 260 HSV-2 and 73 HSV-1 infections were identified. No differences in test performance based on gender, clinic type, location of the lesion, or type of lesion were observed. The sensitivity of HSV-2 detection ranged from 98.4 to 100% depending on the analytical approach, while the specificity ranged from 80.6%, compared to the less sensitive culture method, to 97.0%, compared to PCR. For HSV-1, the sensitivity and specificity ranges were 96.7 to 100% and 95.1 to 99.4%, respectively. This assay may improve our ability to accurately diagnose anogenital lesions due to herpes infection.
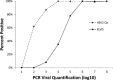
Figures


References
-
- Benedetti J, Corey L, Ashley R. 1994. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann. Intern. Med. 121: 847–854 - PubMed
-
- Centers for Disease Control and Prevention 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb. Mortal. Weekly Rep. 59: 1–112
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

