Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients
- PMID: 22875918
- PMCID: PMC3470811
- DOI: 10.1523/JNEUROSCI.5628-11.2012
Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients
Abstract
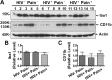
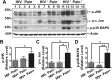
Studies with animal models have suggested that reaction of glia, including microglia and astrocytes, critically contributes to the development and maintenance of chronic pain. However, the involvement of glial reaction in human chronic pain is unclear. We performed analyses to compare the glial reaction profiles in the spinal dorsal horn (SDH) from three cohorts of sex- and age-matched human postmortem tissues: (1) HIV-negative patients, (2) HIV-positive patients without chronic pain, and (3) HIV patients with chronic pain. Our results indicate that the expression levels of CD11b and Iba1, commonly used for labeling microglial cells, did not differ in the three patient groups. However, GFAP and S100β, often used for labeling astrocytes, were specifically upregulated in the SDH of the "pain-positive" HIV patients but not in the "pain-negative" HIV patients. In addition, proinflammatory cytokines, TNFα and IL-1β, were specifically increased in the SDH of pain-positive HIV patients. Furthermore, proteins in the MAPK signaling pathway, including pERK, pCREB and c-Fos, were also upregulated in the SDH of pain-positive HIV patients. Our findings suggest that reaction of astrocytes in the SDH may play a role during the maintenance phase of HIV-associated chronic pain.
Figures






References
-
- Che Y, Yu YM, Han PL, Lee JK. Delayed induction of p38 MAPKs in reactive astrocytes in the brain of mice after KA-induced seizure. Brain Res Mol Brain Res. 2001;94:157–165. - PubMed
-
- Chung IY, Benveniste EN. Tumor necrosis factor-α production by astrocytes. Induction by lipopolysaccharide, IFN-γ, and IL-1β. J Immunol. 1990;144:2999–3007. - PubMed
-
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007a;11:223–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
