Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice
- PMID: 22875966
- PMCID: PMC3457137
- DOI: 10.1128/JVI.01093-12
Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice
Abstract
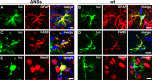
Beta interferon (IFN-β) is a major component of innate immunity in mammals, but information on the in vivo source of this cytokine after pathogen infection is still scarce. To identify the cell types responsible for IFN-β production during viral encephalitis, we used reporter mice that express firefly luciferase under the control of the IFN-β promoter and stained organ sections with luciferase-specific antibodies. Numerous luciferase-positive cells were detected in regions of La Crosse virus (LACV)-infected mouse brains that contained many infected cells. Double-staining experiments with cell-type-specific markers revealed that similar numbers of astrocytes and microglia of infected brains were luciferase positive, whereas virus-infected neurons rarely contained detectable levels of luciferase. Interestingly, if a mutant LACV unable of synthesizing the IFN-antagonistic factor NSs was used for challenge, the vast majority of the IFN-β-producing cells in infected brains were astrocytes rather than microglia. Similar conclusions were reached in a second series of experiments in which conditional reporter mice expressing the luciferase reporter gene solely in defined cell types were infected with wild-type or mutant LACV. Collectively, our data suggest that glial cells rather than infected neurons represent the major source of IFN-β in LACV-infected mouse brains. They further indicate that IFN-β synthesis in astrocytes and microglia is differentially affected by the viral IFN antagonist, presumably due to differences in LACV susceptibility of these two cell types.
Figures






References
-
- Asselin-Paturel C, et al. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150 - PubMed
-
- Bennett RS, et al. 2008. La Crosse virus infectivity, pathogenesis, and immunogenicity in mice and monkeys. Virol. J. 5:25 doi:10.1186/1743-422X-5-25 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

