Filamentous influenza virus enters cells via macropinocytosis
- PMID: 22875971
- PMCID: PMC3457176
- DOI: 10.1128/JVI.05992-11
Filamentous influenza virus enters cells via macropinocytosis
Abstract
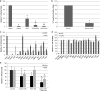
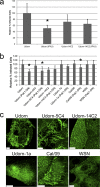
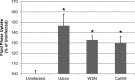
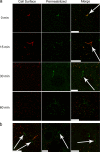
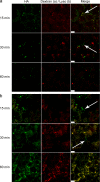
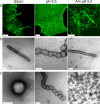
Influenza virus is pleiomorphic, producing both spherical (100-nm-diameter) and filamentous (100-nm by 20-μm) virions. While the spherical virions are known to enter host cells through exploitation of clathrin-mediated endocytosis, the entry pathway for filamentous virions has not been determined, though the existence of an alternative, non-clathrin-, non-caveolin-mediated entry pathway for influenza virus has been known for many years. In this study, we confirm recent results showing that influenza virus utilizes macropinocytosis as an alternate entry pathway. Furthermore, we find that filamentous influenza viruses use macropinocytosis as the primary entry mechanism. Virions enter cells as intact filaments within macropinosomes and are trafficked to the acidic late-endosomal compartment. Low pH triggers a conformational change in the M2 ion channel protein, altering membrane curvature and leading to a fragmentation of the filamentous virions. This fragmentation may enable more-efficient fusion between the viral and endosomal membranes.
Figures


References
-
- Ada GL, Perry BT, Abbot A. 1958. Biological and physical properties of the Ryan strain of filamentous influenza virus. J. Gen. Microbiol. 19:23–39 - PubMed
-
- Alexander A. 1998. Endocytosis and intracellular sorting of receptor tyrosine kinases. Front. Biosci. 3:d729–d738 - PubMed
-
- Bar-Sagi D, Hall A. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227–238 - PubMed
-
- Bourmakina SV, Garcia-Sastre A. 2003. Reverse genetics studies on the filamentous morphology of influenza A virus. J. Gen. Virol. 84:517–527 - PubMed
-
- Burnet FM, Lind PE. 1957. Studies on filamentary forms of influenza virus with special reference to the use of dark-ground-microscopy. Arch. Gesamte Virusforsch. 7:413–422 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

