Drug use evaluation of moxifloxacin (avelox) using a hand-held electronic device at a canadian teaching hospital
- PMID: 22876087
- PMCID: PMC3411225
Drug use evaluation of moxifloxacin (avelox) using a hand-held electronic device at a canadian teaching hospital
Abstract
Background: The use of moxifloxacin (Avelox) has increased at Vancouver General Hospital since its introduction onto the formulary in 2002. It is unclear, however, whether the use of the drug is optimal according to its indication. Hand-held electronic devices, such as personal digital assistants (PDAs), are novel tools that can be used during routine patient care to collect data for drug use evaluation (DUE) reviews.
Objective: We hypothesized that moxifloxacin was over-utilized and that opportunities existed to optimize its use. This study was designed to characterize moxifloxacin use in concordance with evidence-based assessment criteria. The feasibility of using a PDA device as a data-collection tool was also evaluated.
Design: An observational DUE was conducted over a 4-week period (from February 17 to March 16, 2007) at Vancouver General Hospital, a 955-bed tertiary care hospital. Inpatients who received at least one dose of moxifloxacin were enrolled. Evidence-based assessment criteria were developed to evaluate the appropriateness of moxifloxacin use, and a PDA database was developed for data collection. The primary endpoint was the proportion of moxifloxacin use for approved first-line indications.
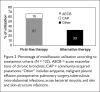
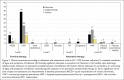
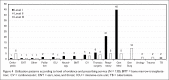
Results: A total of 132 patients were included. Eighty-nine patients (67%) received moxifloxacin for first-line indications, including community-acquired pneumonia (57%) and acute exacerbation of chronic bronchitis (10%). Forty-three patients (33%) had alternative indications, primarily hospital-acquired pneumonia (25%). In 129 evaluable patients, approximately half (51%) of the clinical outcomes were successful; 37% were indeterminate; and 12% were failures. General medicine and respiratory service clinicians prescribed moxifloxacin more appropriately compared with surgical service personnel. Most of the pharmacists supported the use of PDAs as DUE data-collection tools.
Conclusion: Overall, moxifloxacin utilization at Vancouver General Hospital was appropriate according to evidence-based assessment criteria. Additional opportunities to improve its use exist through health care staff education. PDAs are ideal data-collection tools for DUEs, as they can be conveniently used during routine patient care.
Figures

References
-
- Keating GM, Scott LJ. Moxifloxacin: A review of its use in the management of bacterial infections. Drugs. 2004;64(20):2347–2377. - PubMed
-
- Avelox Product Monograph. Toronto, Ontario, Canada: Bayer Health Canada, Inc; Feb 5, 2007.
-
- Speirs GE, Fenelon LE, Reeves DS, et al. An audit of ciprofloxacin use in a district general hospital. J Antimicrob Chemother. 1995;36:201–207. - PubMed
-
- Lacombe K, Cariou S, Tilleul P, et al. Optimizing fluoroquinolone utilization in a public hospital: A prospective study of educational intervention. Eur J Clin Microbiol Infect Dis. 2005;24:6–11. - PubMed
-
- Pulcini C, Mondain V, Lieutier F, et al. Fluoroquinolone prescriptions in a teaching hospital: A prospective audit. Scand J Infect Dis. 2007;39:1013–1017. - PubMed
LinkOut - more resources
Full Text Sources
