Asymmetric Methods for the Synthesis of Flavanones, Chromanones, and Azaflavanones
- PMID: 22876166
- PMCID: PMC3412359
- DOI: 10.1002/ejoc.201101228
Asymmetric Methods for the Synthesis of Flavanones, Chromanones, and Azaflavanones
Abstract
Flavanones, chromanones, and related structures are privileged natural products that display a wide variety of biological activities. Although flavanoids are abundant in nature, there are a limited number of available general and efficient synthetic methods for accessing molecules of this class in a stereoselective manner. Their structurally simple architectures belie the difficulties involved in installation and maintenance of the stereogenic configuration at the C2 position, which can be sensitive and can undergo epimerization under mildly acidic, basic, and thermal reaction conditions. This review presents the methods currently used to access these related structures. The synthetic methods include manipulation of the flavone/flavanone core, carbon-carbon bond formation, and carbon-heteroatom bond formation.
Figures




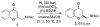
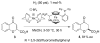
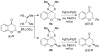
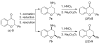







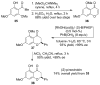






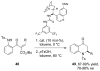
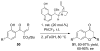




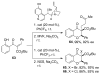
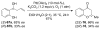
References
-
- Andersen ØM, Markham KR. Flavonoids: Chemistry, Biochemistry and Applications. CRC, Taylor & Francis; Boca Raton, FL: 2006.
-
- Evans BE, Rittle KE, Bock MG, Dipardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. J Med Chem. 1988;31:2235–2246. - PubMed
-
- Veitch NC, Grayer RJ. Nat Prod Rep. 2011;28:1626–1695. - PubMed
- Veitch NC, Grayer REJ. Nat Prod Rep. 2008;25:555–611. - PubMed
- Williams CA, Grayer RJ. Nat Prod Rep. 2004;21:539–573. - PubMed
- Harborne JB, Williams CA. Nat Prod Rep. 2001;18:310–333. - PubMed
- Harborne JB, Williams CA. Nat Prod Rep. 1998;15:631–652.
- Harborne JB, Williams CA. Nat Prod Rep. 1995;12:639–657.
- Crozier A, Jaganath IB, Clifford MN. Nat Prod Rep. 2009;26:1001–1043. - PubMed
- Crozier A, Del Rio D, Clifford MN. Mol Aspects Med. 2010;31:446–467. - PubMed
- Silva FRMB, Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MSRB, Folador P, Damazio RG, Pizzolatti MG. Mini-Rev Med Chem. 2008;8:1429–1440. - PubMed
- Veitch NC. Nat Prod Rep. 2009;26:776–802. - PubMed
- Veitch NC. Nat Prod Rep. 2007;24:417–464. - PubMed
- Reynaud J, Guilet D, Terreux R, Lussignol M, Walchshofer N. Nat Prod Rep. 2005;22:504–515. - PubMed
-
- Robards K, Tucker G. Crit Rev Food Sci Nutr Crit Rev Food Sci. 2008;48:929–966. - PubMed
- Boland GM, Donnelly DMX. Nat Prod Rep. 1998;15:241–260.
- Daayf F, Lattanzio V, Santos-Buelga C, Escribano-Bailon MT. Recent Advances in Polyphenol Research. Wiley-Blackwell; Oxford, Ames, Iowa: 2008.
- Keller RB. Flavonoids: Biosynthesis, Biological Effects and Dietary Sources. Nova Science Publishers; Hauppauge, NY: 2009.
- Huang YB, Du H, Tang YX. Appl Microbiol Biotechnol. 2010;86:1293–1312. - PubMed
-
- Dittmer C, Raabe G, Hintermann L. Eur J Org Chem. 2007:5886–5898.
- Wang YC, Chen S, Yu O. Appl Microbiol Biotechnol. 2011;91:949–956. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
