Structural and biochemical basis for development of influenza virus inhibitors targeting the PA endonuclease
- PMID: 22876176
- PMCID: PMC3410894
- DOI: 10.1371/journal.ppat.1002830
Structural and biochemical basis for development of influenza virus inhibitors targeting the PA endonuclease
Abstract
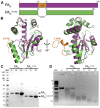
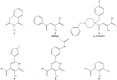
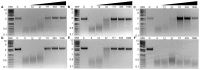
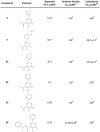
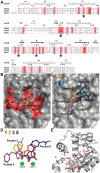
Emerging influenza viruses are a serious threat to human health because of their pandemic potential. A promising target for the development of novel anti-influenza therapeutics is the PA protein, whose endonuclease activity is essential for viral replication. Translation of viral mRNAs by the host ribosome requires mRNA capping for recognition and binding, and the necessary mRNA caps are cleaved or "snatched" from host pre-mRNAs by the PA endonuclease. The structure-based development of inhibitors that target PA endonuclease is now possible with the recent crystal structure of the PA catalytic domain. In this study, we sought to understand the molecular mechanism of inhibition by several compounds that are known or predicted to block endonuclease-dependent polymerase activity. Using an in vitro endonuclease activity assay, we show that these compounds block the enzymatic activity of the isolated PA endonuclease domain. Using X-ray crystallography, we show how these inhibitors coordinate the two-metal endonuclease active site and engage the active site residues. Two structures also reveal an induced-fit mode of inhibitor binding. The structures allow a molecular understanding of the structure-activity relationship of several known influenza inhibitors and the mechanism of drug resistance by a PA mutation. Taken together, our data reveal new strategies for structure-based design and optimization of PA endonuclease inhibitors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
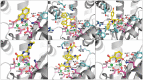


References
-
- Reid AH, Taubenberger JK, Fanning TG. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 2001;3:81–87. - PubMed
-
- WHO. Avian influenza in humans. 2012. Available: http://www.who.int/csr/disease/avian_influenza/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

