CPAF: a Chlamydial protease in search of an authentic substrate
- PMID: 22876181
- PMCID: PMC3410858
- DOI: 10.1371/journal.ppat.1002842
CPAF: a Chlamydial protease in search of an authentic substrate
Abstract
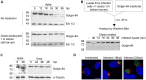
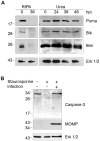
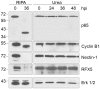
Bacteria in the genus Chlamydia are major human pathogens that cause an intracellular infection. A chlamydial protease, CPAF, has been proposed as an important virulence factor that cleaves or degrades at least 16 host proteins, thereby altering multiple cellular processes. We examined 11 published CPAF substrates and found that there was no detectable proteolysis when CPAF activity was inhibited during cell processing. We show that the reported proteolysis of these putative CPAF substrates was due to enzymatic activity in cell lysates rather than in intact cells. Nevertheless, Chlamydia-infected cells displayed Chlamydia-host interactions, such as Golgi reorganization, apoptosis resistance, and host cytoskeletal remodeling, that have been attributed to CPAF-dependent proteolysis of host proteins. Our findings suggest that other mechanisms may be responsible for these Chlamydia-host interactions, and raise concerns about all published CPAF substrates and the proposed roles of CPAF in chlamydial pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Reassessing the role of the secreted protease CPAF in Chlamydia trachomatis infection through genetic approaches.Pathog Dis. 2014 Aug;71(3):336-51. doi: 10.1111/2049-632X.12179. Epub 2014 May 16. Pathog Dis. 2014. PMID: 24838663 Free PMC article.
-
Chlamydia trachomatis outer membrane complex protein B (OmcB) is processed by the protease CPAF.J Bacteriol. 2013 Mar;195(5):951-7. doi: 10.1128/JB.02087-12. Epub 2012 Dec 7. J Bacteriol. 2013. PMID: 23222729 Free PMC article.
-
Golgi fragmentation and sphingomyelin transport to Chlamydia trachomatis during penicillin-induced persistence do not depend on the cytosolic presence of the chlamydial protease CPAF.PLoS One. 2014 Jul 28;9(7):e103220. doi: 10.1371/journal.pone.0103220. eCollection 2014. PLoS One. 2014. PMID: 25068694 Free PMC article.
-
[Effector proteins of Clamidia].Mol Biol (Mosk). 2009 Nov-Dec;43(6):963-83. Mol Biol (Mosk). 2009. PMID: 20088373 Review. Russian.
-
[Chlamydia trachomatis proteasome protein as one of the significant pathogenicity factors of exciter].Mol Gen Mikrobiol Virusol. 2014;(2):3-8. Mol Gen Mikrobiol Virusol. 2014. PMID: 25080811 Review. Russian.
Cited by
-
Protochlamydia induces apoptosis of human HEp-2 cells through mitochondrial dysfunction mediated by chlamydial protease-like activity factor.PLoS One. 2013;8(2):e56005. doi: 10.1371/journal.pone.0056005. Epub 2013 Feb 11. PLoS One. 2013. PMID: 23409113 Free PMC article.
-
Chlamydia trachomatis Alters Mitochondrial Protein Composition and Secretes Effector Proteins That Target Mitochondria.mSphere. 2022 Dec 21;7(6):e0042322. doi: 10.1128/msphere.00423-22. Epub 2022 Oct 26. mSphere. 2022. PMID: 36286535 Free PMC article.
-
Characterization of CPAF critical residues and secretion during Chlamydia trachomatis infection.Infect Immun. 2015 Jun;83(6):2234-41. doi: 10.1128/IAI.00275-15. Epub 2015 Mar 16. Infect Immun. 2015. PMID: 25776755 Free PMC article.
-
An Ancient Molecular Arms Race: Chlamydia vs. Membrane Attack Complex/Perforin (MACPF) Domain Proteins.Front Immunol. 2020 Jul 14;11:1490. doi: 10.3389/fimmu.2020.01490. eCollection 2020. Front Immunol. 2020. PMID: 32760406 Free PMC article. Review.
-
Chlamydia trachomatis Whole-Proteome Microarray Analysis of The Netherlands Chlamydia Cohort Study.Microorganisms. 2019 Dec 16;7(12):703. doi: 10.3390/microorganisms7120703. Microorganisms. 2019. PMID: 31888186 Free PMC article.
References
-
- CDC. Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:1–100. - PubMed
-
- Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. Washington, D.C.: American Society for Microbiology; 1999. pp. 139–169. editor.
-
- Blasi F, Tarsia P, Aliberti S. Chlamydophila pneumoniae. Clin Microbiol Infect. 2009;15:29–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

