UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph polarize F-actin during embryonic morphogenesis by regulating the WAVE/SCAR actin nucleation complex
- PMID: 22876199
- PMCID: PMC3410845
- DOI: 10.1371/journal.pgen.1002863
UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph polarize F-actin during embryonic morphogenesis by regulating the WAVE/SCAR actin nucleation complex
Abstract
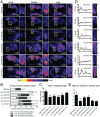
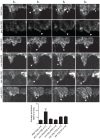
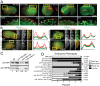
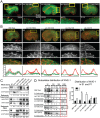
Many cells in a developing embryo, including neurons and their axons and growth cones, must integrate multiple guidance cues to undergo directed growth and migration. The UNC-6/netrin, SLT-1/slit, and VAB-2/Ephrin guidance cues, and their receptors, UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph, are known to be major regulators of cellular growth and migration. One important area of research is identifying the molecules that interpret this guidance information downstream of the guidance receptors to reorganize the actin cytoskeleton. However, how guidance cues regulate the actin cytoskeleton is not well understood. We report here that UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph differentially regulate the abundance and subcellular localization of the WAVE/SCAR actin nucleation complex and its activator, Rac1/CED-10, in the Caenorhabditis elegans embryonic epidermis. Loss of any of these three pathways results in embryos that fail embryonic morphogenesis. Similar defects in epidermal enclosure have been observed when CED-10/Rac1 or the WAVE/SCAR actin nucleation complex are missing during embryonic development in C. elegans. Genetic and molecular experiments demonstrate that in fact, these three axonal guidance proteins differentially regulate the levels and membrane enrichment of the WAVE/SCAR complex and its activator, Rac1/CED-10, in the epidermis. Live imaging of filamentous actin (F-actin) in embryos developing in the absence of individual guidance receptors shows that high levels of F-actin are not essential for polarized cell migrations, but that properly polarized distribution of F-actin is essential. These results suggest that proper membrane recruitment and activation of CED-10/Rac1 and of WAVE/SCAR by signals at the plasma membrane result in polarized F-actin that permits directed movements and suggest how multiple guidance cues can result in distinct changes in actin nucleation during morphogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis of C. elegans. Neuron. 1990;4:61–85. - PubMed
-
- Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. - PubMed
-
- Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–27. - PubMed
-
- Zallen JA, Kirch SA, Bargmann CI. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development. 1999;126:3679–92. - PubMed
-
- Boulin T, Pocock R, Hobert O. A novel Eph receptor-interacting IgSF protein provides C. elegans motorneurons with midline guidepost function. Curr Biol. 2006;16:1871–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

