Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system
- PMID: 22876201
- PMCID: PMC3410851
- DOI: 10.1371/journal.pgen.1002866
Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system
Abstract
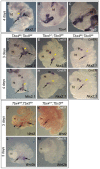
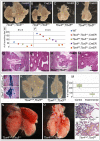
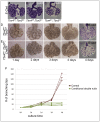
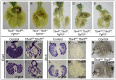
Normal development of the respiratory system is essential for survival and is regulated by multiple genes and signaling pathways. Both Tbx4 and Tbx5 are expressed throughout the mesenchyme of the developing lung and trachea; and, although multiple genes are known to be required in the epithelium, only Fgfs have been well studied in the mesenchyme. In this study, we investigated the roles of Tbx4 and Tbx5 in lung and trachea development using conditional mutant alleles and two different Cre recombinase transgenic lines. Loss of Tbx5 leads to a unilateral loss of lung bud specification and absence of tracheal specification in organ culture. Mutants deficient in Tbx4 and Tbx5 show severely reduced lung branching at mid-gestation. Concordant with this defect, the expression of mesenchymal markers Wnt2 and Fgf10, as well as Fgf10 target genes Bmp4 and Spry2, in the epithelium is downregulated. Lung branching undergoes arrest ex vivo when Tbx4 and Tbx5 are both completely lacking. Lung-specific Tbx4 heterozygous;Tbx5 conditional null mice die soon after birth due to respiratory distress. These pups have small lungs and show severe disruptions in tracheal/bronchial cartilage rings. Sox9, a master regulator of cartilage formation, is expressed in the trachea; but mesenchymal cells fail to condense and consequently do not develop cartilage normally at birth. Tbx4;Tbx5 double heterozygous mutants show decreased lung branching and fewer tracheal cartilage rings, suggesting a genetic interaction. Finally, we show that Tbx4 and Tbx5 interact with Fgf10 during the process of lung growth and branching but not during tracheal/bronchial cartilage development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Hum Mol Genet. 2004;13 Spec No 2:R207–215. - PubMed
-
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. - PubMed
-
- Spooner BS, Wessells NK. Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J Exp Zool. 1970;175:445–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

