The KCNE Tango - How KCNE1 Interacts with Kv7.1
- PMID: 22876232
- PMCID: PMC3410610
- DOI: 10.3389/fphar.2012.00142
The KCNE Tango - How KCNE1 Interacts with Kv7.1
Abstract
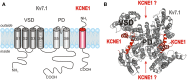
The classical tango is a dance characterized by a 2/4 or 4/4 rhythm in which the partners dance in a coordinated way, allowing dynamic contact. There is a surprising similarity between the tango and how KCNE β-subunits "dance" to the fast rhythm of the cell with their partners from the Kv channel family. The five KCNE β-subunits interact with several members of the Kv channels, thereby modifying channel gating via the interaction of their single transmembrane-spanning segment, the extracellular amino terminus, and/or the intracellular carboxy terminus with the Kv α-subunit. Best studied is the molecular basis of interactions between KCNE1 and Kv7.1, which, together, supposedly form the native cardiac I(Ks) channel. Here we review the current knowledge about functional and molecular interactions of KCNE1 with Kv7.1 and try to summarize and interpret the tango of the KCNEs.
Keywords: KCNE1; Kv channel; Kv7.1; β-subunit.
Figures

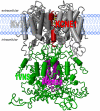

References
-
- Aggeli A., Bannister M. L., Bell M., Boden N., Findlay J. B., Hunter M., Knowles P. F., Yang J. C. (1998). Conformation and ion-channeling activity of a 27-residue peptide modeled on the single-transmembrane segment of the IsK (minK) protein. Biochemistry 37, 8121–8131 10.1021/bi972112h - DOI - PubMed
-
- Arrighi I., Bloch-Faure M., Grahammer F., Bleich M., Warth R., Mengual R., Drici M. D., Barhanin J., Meneton P. (2001). Altered potassium balance and aldosterone secretion in a mouse model of human congenital long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 98, 8792–8797 10.1073/pnas.141233398 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

