Phosphonate analogs of 2-oxoglutarate perturb metabolism and gene expression in illuminated Arabidopsis leaves
- PMID: 22876250
- PMCID: PMC3410613
- DOI: 10.3389/fpls.2012.00114
Phosphonate analogs of 2-oxoglutarate perturb metabolism and gene expression in illuminated Arabidopsis leaves
Abstract
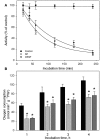
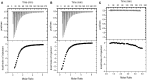
Although the role of the 2-oxoglutarate dehydrogenase complex (2-OGDHC) has previously been demonstrated in plant heterotrophic tissues its role in photosynthetically active tissues remains poorly understood. By using a combination of metabolite and transcript profiles we here investigated the function of 2-OGDHC in leaves of Arabidopsis thaliana via use of specific phosphonate inhibitors of the enzyme. Incubation of leaf disks with the inhibitors revealed that they produced the anticipated effects on the in situ enzyme activity. In vitro experiments revealed that succinyl phosphonate (SP) and a carboxy ethyl ester of SP are slow-binding inhibitors of the 2-OGDHC. Our results indicate that the reduced respiration rates are associated with changes in the regulation of metabolic and signaling pathways leading to an imbalance in carbon-nitrogen metabolism and cell homeostasis. The inducible alteration of primary metabolism was associated with altered expression of genes belonging to networks of amino acids, plant respiration, and sugar metabolism. In addition, by using isothermal titration calorimetry we excluded the possibility that the changes in gene expression resulted from an effect on 2-oxoglutarate (2OG) binding to the carbon/ATP sensing protein PII. We also demonstrated that the 2OG degradation by the 2-oxoglutarate dehydrogenase strongly influences the distribution of intermediates of the tricarboxylic acid (TCA) cycle and the GABA shunt. Our results indicate that the TCA cycle activity is clearly working in a non-cyclic manner upon 2-OGDHC inhibition during the light period.
Keywords: 2-oxoglutarate; 2-oxoglutarate dehydrogenase; GABA; PII; TCA cycle; phosphonate inhibitors.
Figures






References
-
- Alhagdow M., Mounet F., Gilbert L., Nunes-Nesi A., Garcia V., Just D., Petit J., Beauvoit B., Fernie A. R., Rothan C., Baldet P. (2007). Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 145, 1408–142210.1104/pp.107.106500 - DOI - PMC - PubMed
-
- Araújo W. L., Ishizaki K., Nunes-Nesi A., Larson T. R., Tohge T., Krahnert I., Witt S., Obata T., Schauer N., Graham I. A., Leaver C. J., Fernie A. R. (2010). Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 22, 1549–156310.1105/tpc.110.075630 - DOI - PMC - PubMed
-
- Araújo W. L., Nunes-Nesi A., Trenkamp S., Bunik V. I., Fernie A. R. (2008). Inhibition of 2-oxoglutarate dehydrogenase in potato tuber suggests the enzyme is limiting for respiration and confirms its importance in nitrogen assimilation. Plant Physiol. 148, 1782–179610.1104/pp.108.126219 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

