ER Import Sites and Their Relationship to ER Exit Sites: A New Model for Bidirectional ER-Golgi Transport in Higher Plants
- PMID: 22876251
- PMCID: PMC3410614
- DOI: 10.3389/fpls.2012.00143
ER Import Sites and Their Relationship to ER Exit Sites: A New Model for Bidirectional ER-Golgi Transport in Higher Plants
Abstract
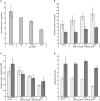
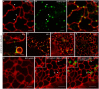
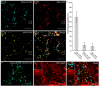
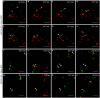
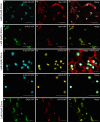
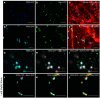
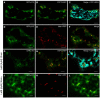
Per definition, ER exit sites are COPII vesiculation events at the surface of the ER and in higher plants are only visualizable in the electron microscope through cryofixation techniques. Fluorescent COPII labeling moves with Golgi stacks and locates to the interface between the ER and the Golgi. In contrast, the domain of the ER where retrograde COPI vesicles fuse, i.e., ER import sites (ERIS), has remained unclear. To identify ERIS we have employed ER-located SNAREs and tethering factors. We screened several SNAREs (SYP81, the SYP7 family, and USE1) to find a SNARE whose overexpression did not disrupt ER-Golgi traffic and which gave rise to discrete fluorescent punctae when expressed with an XFP tag. Only the Qc-SNARE SYP72 fulfilled these criteria. When coexpressed with SYP72-YFP, both the type I-membrane protein RFP-p24δ5 and the luminal marker CFP-HDEL whose ER localization are due to an efficient COPI-mediated recycling, form nodules along the tubular ER network. SYP72-YFP colocalizes with these nodules which are not seen when RFP-p24δ5 or CFP-HDEL is expressed alone or when SYP72-YFP is coexpressed with a mutant form of RFP-p24δ5 that cannot exit the ER. SYP72-YFP does not colocalize with Golgi markers, except when the Golgi stacks are immobilized through actin depolymerization. Endogenous SYP7 SNAREs, also colocalize with immobilized COPII/Golgi. In contrast, XFP-tagged versions of plant homologs to TIP20 of the Dsl1 COPI-tethering factor complex, and the COPII-tethering factor p115 colocalize perfectly with Golgi stacks irrespective of the motile status. These data suggest that COPI vesicle fusion with the ER is restricted to periods when Golgi stacks are stationary, but that when moving both COPII and COPI vesicles are tethered and collect in the ER-Golgi interface. Thus, the Golgi stack and an associated domain of the ER thereby constitute a mobile secretory and recycling unit: a unique feature in eukaryotic cells.
Keywords: ER export sites; ER import sites; Golgi motility; SNAREs; secretory unit; tobacco.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

