Specialized motor-driven dusp1 expression in the song systems of multiple lineages of vocal learning birds
- PMID: 22876306
- PMCID: PMC3410896
- DOI: 10.1371/journal.pone.0042173
Specialized motor-driven dusp1 expression in the song systems of multiple lineages of vocal learning birds
Abstract
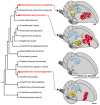
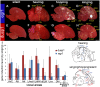
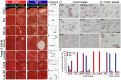
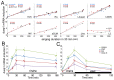
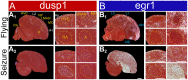
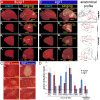
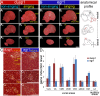
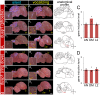
Mechanisms for the evolution of convergent behavioral traits are largely unknown. Vocal learning is one such trait that evolved multiple times and is necessary in humans for the acquisition of spoken language. Among birds, vocal learning is evolved in songbirds, parrots, and hummingbirds. Each time similar forebrain song nuclei specialized for vocal learning and production have evolved. This finding led to the hypothesis that the behavioral and neuroanatomical convergences for vocal learning could be associated with molecular convergence. We previously found that the neural activity-induced gene dual specificity phosphatase 1 (dusp1) was up-regulated in non-vocal circuits, specifically in sensory-input neurons of the thalamus and telencephalon; however, dusp1 was not up-regulated in higher order sensory neurons or motor circuits. Here we show that song motor nuclei are an exception to this pattern. The song nuclei of species from all known vocal learning avian lineages showed motor-driven up-regulation of dusp1 expression induced by singing. There was no detectable motor-driven dusp1 expression throughout the rest of the forebrain after non-vocal motor performance. This pattern contrasts with expression of the commonly studied activity-induced gene egr1, which shows motor-driven expression in song nuclei induced by singing, but also motor-driven expression in adjacent brain regions after non-vocal motor behaviors. In the vocal non-learning avian species, we found no detectable vocalizing-driven dusp1 expression in the forebrain. These findings suggest that independent evolutions of neural systems for vocal learning were accompanied by selection for specialized motor-driven expression of the dusp1 gene in those circuits. This specialized expression of dusp1 could potentially lead to differential regulation of dusp1-modulated molecular cascades in vocal learning circuits.
Conflict of interest statement
Figures


Similar articles
-
The dusp1 immediate early gene is regulated by natural stimuli predominantly in sensory input neurons.J Comp Neurol. 2010 Jul 15;518(14):2873-901. doi: 10.1002/cne.22370. J Comp Neurol. 2010. PMID: 20506480 Free PMC article.
-
Convergent differential regulation of parvalbumin in the brains of vocal learners.PLoS One. 2012;7(1):e29457. doi: 10.1371/journal.pone.0029457. Epub 2012 Jan 6. PLoS One. 2012. PMID: 22238614 Free PMC article.
-
Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin.PLoS One. 2008 Mar 12;3(3):e1768. doi: 10.1371/journal.pone.0001768. PLoS One. 2008. PMID: 18335043 Free PMC article.
-
Neurochemical specializations associated with vocal learning and production in songbirds and budgerigars.Brain Behav Evol. 1994;44(4-5):234-46. doi: 10.1159/000113579. Brain Behav Evol. 1994. PMID: 7842283 Review.
-
The singer and the song: the neuromechanics of avian sound production.Curr Opin Neurobiol. 2014 Oct;28:172-8. doi: 10.1016/j.conb.2014.07.022. Epub 2014 Aug 28. Curr Opin Neurobiol. 2014. PMID: 25171107 Review.
Cited by
-
Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates.Front Evol Neurosci. 2012 Aug 16;4:12. doi: 10.3389/fnevo.2012.00012. eCollection 2012. Front Evol Neurosci. 2012. PMID: 22912615 Free PMC article.
-
Controlling for activity-dependent genes and behavioral states is critical for determining brain relationships within and across species.J Comp Neurol. 2021 Aug;529(12):3206-3221. doi: 10.1002/cne.25157. Epub 2021 May 4. J Comp Neurol. 2021. PMID: 33855704 Free PMC article.
-
Inception of memories that guide vocal learning in the songbird.Science. 2019 Oct 4;366(6461):83-89. doi: 10.1126/science.aaw4226. Science. 2019. PMID: 31604306 Free PMC article.
-
Differential contributions of basal ganglia and thalamus to song initiation, tempo, and structure.J Neurophysiol. 2014 Jan;111(2):248-57. doi: 10.1152/jn.00584.2012. Epub 2013 Oct 30. J Neurophysiol. 2014. PMID: 24174647 Free PMC article.
-
Core and Shell Song Systems Unique to the Parrot Brain.PLoS One. 2015 Jun 24;10(6):e0118496. doi: 10.1371/journal.pone.0118496. eCollection 2015. PLoS One. 2015. PMID: 26107173 Free PMC article.
References
-
- Arendt J, Reznick D (2008) Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Trends Ecol Evol 23: 26–32. - PubMed
-
- Prud'homme B, Gompel N, Rokas A, Kassner VA, Williams TM, et al. (2006) Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440: 1050–1053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

