Transgene therapy for rat anti-Thy1.1 glomerulonephritis via mesangial cell vector with a polyethylenimine/decorin nanocomplex
- PMID: 22876812
- PMCID: PMC3629717
- DOI: 10.1186/1556-276X-7-451
Transgene therapy for rat anti-Thy1.1 glomerulonephritis via mesangial cell vector with a polyethylenimine/decorin nanocomplex
Abstract
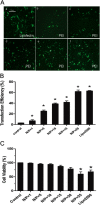
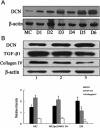
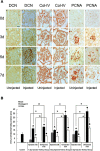
Polyethylenimine (PEI), a cationic polymer, is one of the most efficient non-viral vectors for transgene therapy. Decorin (DCN), a leucine-rich proteoglycan secreted by glomerular mesangial cells (MC), is a promising anti-fibrotic agent for the treatment of glomerulonephritis. In this study, we used PEI-DCN nanocomplexes with different N/P ratios to transfect MC in vitro and deliver the MC vector with PEI-DCN expressing into rat anti-Thy1.1 nephritis kidney tissue via injection into the left renal artery in vivo. The PEI-plasmid DNA complex at N/P 20 had the highest level of transfection efficiency and the lowest level of cytotoxicity in cultured MC. Following injection, the ex vivo gene was transferred successfully into the glomeruli of the rat anti-Thy1.1 nephritis model by the MC vector with the PEI-DCN complex. The exogenous MC with DCN expression was located mainly in the mesangium and the glomerular capillary. Over-expression of DCN in diseased glomeruli could result in the inhibition of collagen IV deposition and MC proliferation. The pathological changes of rat nephritis were alleviated following injection of the vector. These findings demonstrate that the DCN gene delivered by the PEI-DNA nanocomplex with the MC vector is a promising therapeutic method for the treatment of glomerulonephritis.
Figures


Similar articles
-
Usp2-69 overexpression slows down the progression of rat anti-Thy1.1 nephritis.Exp Mol Pathol. 2016 Oct;101(2):249-258. doi: 10.1016/j.yexmp.2016.09.005. Epub 2016 Sep 15. Exp Mol Pathol. 2016. PMID: 27640956
-
Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis.Exp Mol Pathol. 2005 Feb;78(1):17-24. doi: 10.1016/j.yexmp.2004.07.006. Exp Mol Pathol. 2005. PMID: 15596056
-
[The antagonistic effect on anti-thy-1 serum-induced nephritis of rats injected by decorin-transfected mesangial cells vector].Zhonghua Bing Li Xue Za Zhi. 2003 Oct;32(5):444-8. Zhonghua Bing Li Xue Za Zhi. 2003. PMID: 14633458 Chinese.
-
Alpha8 integrin in glomerular mesangial cells and in experimental glomerulonephritis.Kidney Int. 1999 Oct;56(4):1468-80. doi: 10.1046/j.1523-1755.1999.00662.x. Kidney Int. 1999. PMID: 10504498
-
Effect of simvastatin on proliferative nephritis and cell-cycle protein expression.Kidney Int Suppl. 1999 Jul;71:S84-7. doi: 10.1046/j.1523-1755.1999.07121.x. Kidney Int Suppl. 1999. PMID: 10412745 Review.
Cited by
-
Decorin gene upregulation mediated by an adeno-associated virus vector increases intratumoral uptake of nab-paclitaxel in neuroblastoma via inhibition of stabilin-1.Invest New Drugs. 2017 Oct;35(5):566-575. doi: 10.1007/s10637-017-0477-5. Epub 2017 Jun 20. Invest New Drugs. 2017. PMID: 28631095
-
Key roles for the small leucine-rich proteoglycans in renal and pulmonary pathophysiology.Biochim Biophys Acta. 2014 Aug;1840(8):2460-70. doi: 10.1016/j.bbagen.2014.01.035. Epub 2014 Feb 5. Biochim Biophys Acta. 2014. PMID: 24508120 Free PMC article. Review.
-
Gene therapy and kidney diseases.Mol Ther Methods Clin Dev. 2024 Sep 6;32(4):101333. doi: 10.1016/j.omtm.2024.101333. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39434922 Free PMC article. Review.
-
A drug delivery system of HIF-1α siRNA nanoparticles loaded by mesenchymal stem cells on choroidal neovascularization.Nanomedicine (Lond). 2024;19(26):2171-2185. doi: 10.1080/17435889.2024.2393075. Epub 2024 Sep 3. Nanomedicine (Lond). 2024. PMID: 39225143 Free PMC article.
References
-
- Song S, Goudy K, Campbell-Thompson M, Wasserfall C, Scott-Jorgensen M, Wang J, Tang Q, Crawford JM, Ellis TM, Atkinson MA, Flotte TR. Recombinant adeno-associated virusmediated alpha-1 antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther. 2004;7:181–186. doi: 10.1038/sj.gt.3302156. - DOI - PubMed
-
- Lemkine GF, Demeneix BA. Polyethylenimines for in vivo gene delivery. Curr Opin Mol Ther. 2001;7:178–182. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

